ONLINE INQUIRY
Mouse Bronchial Smooth Muscle Cells
Cat.No.: CSC-C9326W
Species: Mouse
Source: Bronchus
Morphology: Multipolar
Cell Type: Smooth Muscle Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Mouse BSMs, or airway smooth muscle cells (ASMs), originate from mesenchymal precursors in the embryo, parallel to epithelial buds, and play a key role in developing the airway tree. BSMs reside at the edges of the bronchus epithelial and lamina propria layers, the smooth muscle layer of the airway wall. In vitro, most BSMs are spindle-shaped with ovoid nuclei located at the middle of the cell. At low cell density, these cells often interweave to form a network, whereas, at high density, they align in a swirl or fence-like pattern.
The primary function of BSMs is to maintain bronchial tone by contracting and relaxing in response to various internal and external stimuli, such as neurotransmitters, hormones, and local chemical agents. This activity narrows the bronchi and maintains the flow of air. Moreover, BSMs also act as immunomodulators by secreting pro-inflammatory factors such as transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), and various cytokines like IL-1, IL-5, IL-6, IL-8 and IL-17. These mediators are essential to inflammatory responses, regulating the movement and proliferation of immune cells, and assisting in the immune defense and repair of the bronchus. BSMs are associated with a variety of lung conditions, including asthma, COPD, and bronchiectasis. Airway smooth muscle cells' contractile activity and mass changes can induce airway inflammation, hyperresponsiveness, and remodelling. Consequently, BSMs are commonly used in model studies of airway hyperresponsiveness diseases. These models are instrumental in elucidating the mechanisms of airway contraction and the changes under pathological conditions.
Exceptionally High Expression of BKγ in mBSMCs.
The largeconductance Ca2+ activated K+ (BK) channel in smooth muscle cells (SMCs) regulates intracellular Ca2+ by negative feedback. BK channels, made up of the (BK) and (BK) subunits, play an important role in regulating muscle tone, though its physiological functions in SMCs are unclear.
Noda's team tested the BK1 activity of BK channels in mouse bronchial smooth muscle cells (mBSMCs). They first evaluated the expression pattern of BK in SM tissues via real-time quantitative PCR and Western blot. They found that BK1 is highly expressed in mouse mBSM, but not in SM tissues such as the aorta, portal vein, vas deferens, bladder, and uterus (Fig. 1A and B). Yet BK and BK1 mRNAs were found in all of the SM tissues tested. BKγ2, BKγ3, and BKγ4 mRNAs were negligible across these tissues, including mBSM (Fig. 1A). Notably, BKγ1 expression was higher in the bronchus compared to the trachea (Fig. 1C). Even after removing airway epithelial cells, BKγ1 mRNA levels in bronchial tissue remained unchanged. Western blotting confirmed abundant BKγ1 protein in mBSM but low levels in mouse aortic smooth muscle (mASM) (Fig. 1D). Immunostaining also revealed BKγ1 colocalization with BKα in the plasma membrane of mBSMCs but not in mASMCs (Fig. 1E). These initial results show that, among various types of mouse SMCs, high expression of BKγ1 was specifically detected in mBSM.
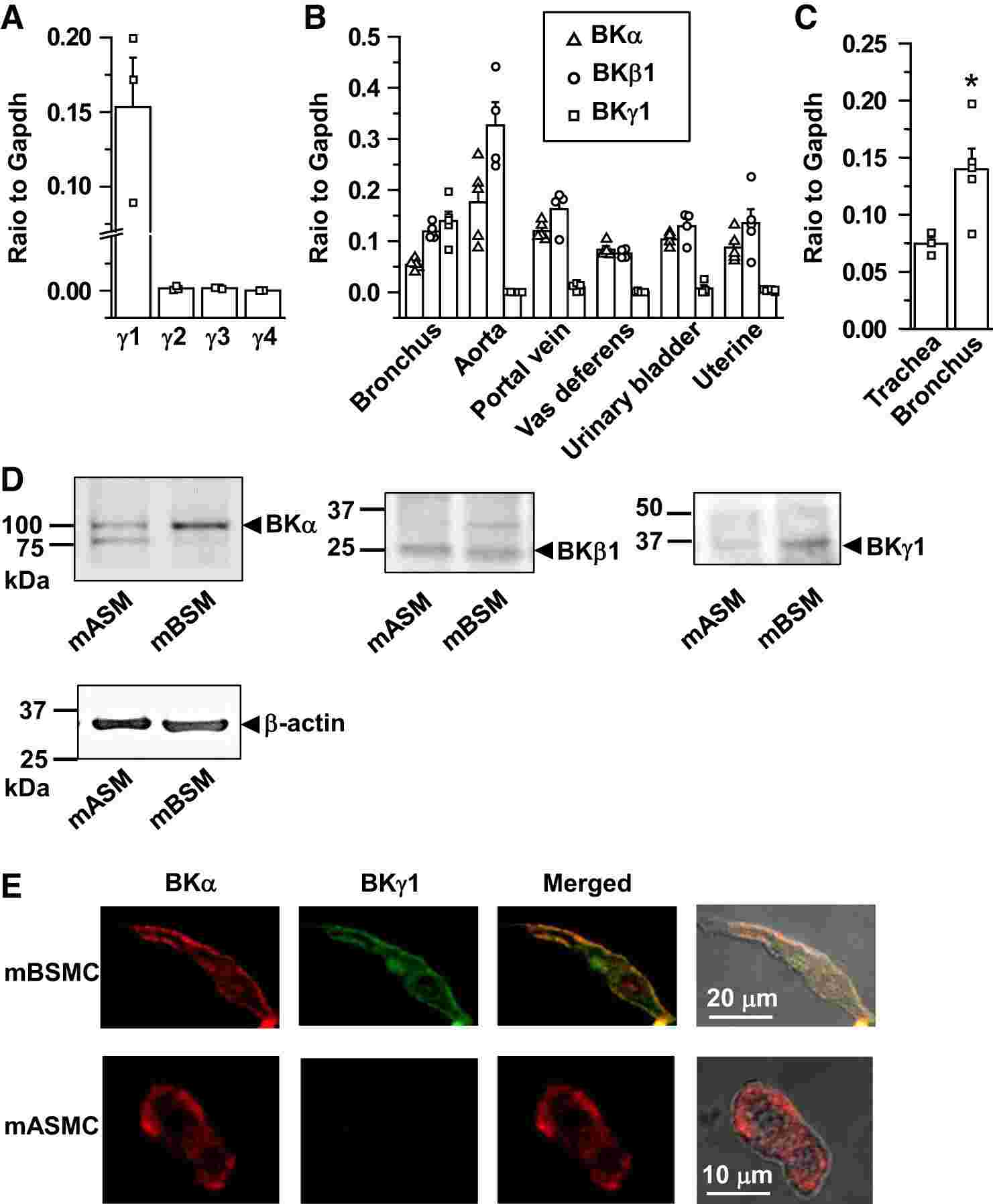 Fig. 1. Large-conductance Ca2+-activated K+ channel γ1-subunit (BKγ1) expression is selectively high in mouse bronchial smooth muscle (mBSM) (Noda S, Suzuki Y, et al., 2019).
Fig. 1. Large-conductance Ca2+-activated K+ channel γ1-subunit (BKγ1) expression is selectively high in mouse bronchial smooth muscle (mBSM) (Noda S, Suzuki Y, et al., 2019).
Effects of Epac Regulators on the Proliferation and Apoptosis of ASMCs
β2 receptor agonists induce airway smooth muscle relaxation by increasing intracellular cAMP production. PKA is the traditional downstream signaling pathway of cAMP. Exchange protein directly activated by cAMP (Epac) was identified as another important signaling molecule of cAMP recently. The role of Epac in asthmatic airway inflammation and airway remodeling is unclear.
Chen et al. established OVA-sensitized and -challenged acute and chronic asthma mice models and demonstrated that Epac activator and inhibitor had opposite effects on airway inflammation and airway remodeling. Then, they investigated the effects of Epac regulators on ASMCs proliferation and apoptosis. They used the CCK-8 assay and Annexin-V labeling to examine mouse airway smooth muscle cells (ASMCs) treated with 8pCPT (an Epac-selective cAMP analog, 8-pCPT-2'-O-Me-cAMP) and ESI-09 (an Epac inhibitor). The ASMCs are bronchial smooth muscle cells isolated from mouse bronchi. The results showed that, 8pCPT inhibited ASMCs proliferation in a dose-dependent manner at 24, 48, and 72 hours. ESI-09 increased ASMCs proliferation at 10 and 100 μM (Fig. 2a). 8pCPT promoted ASMCs apoptosis at 10 and 100 μM after 48 hours, while ESI-09 had no effect (Fig. 2b). Caspase-3 expression increased, and survivin expression decreased in ASMCs treated with 8pCPT for 48 hours at both mRNA and protein levels. ESI-09 did not affect caspase-3 or survivin expression (Fig. 2c, d). These findings indicate 8pCPT inhibits ASMCs proliferation and promotes apoptosis, whereas ESI-09 promotes proliferation without affecting apoptosis.
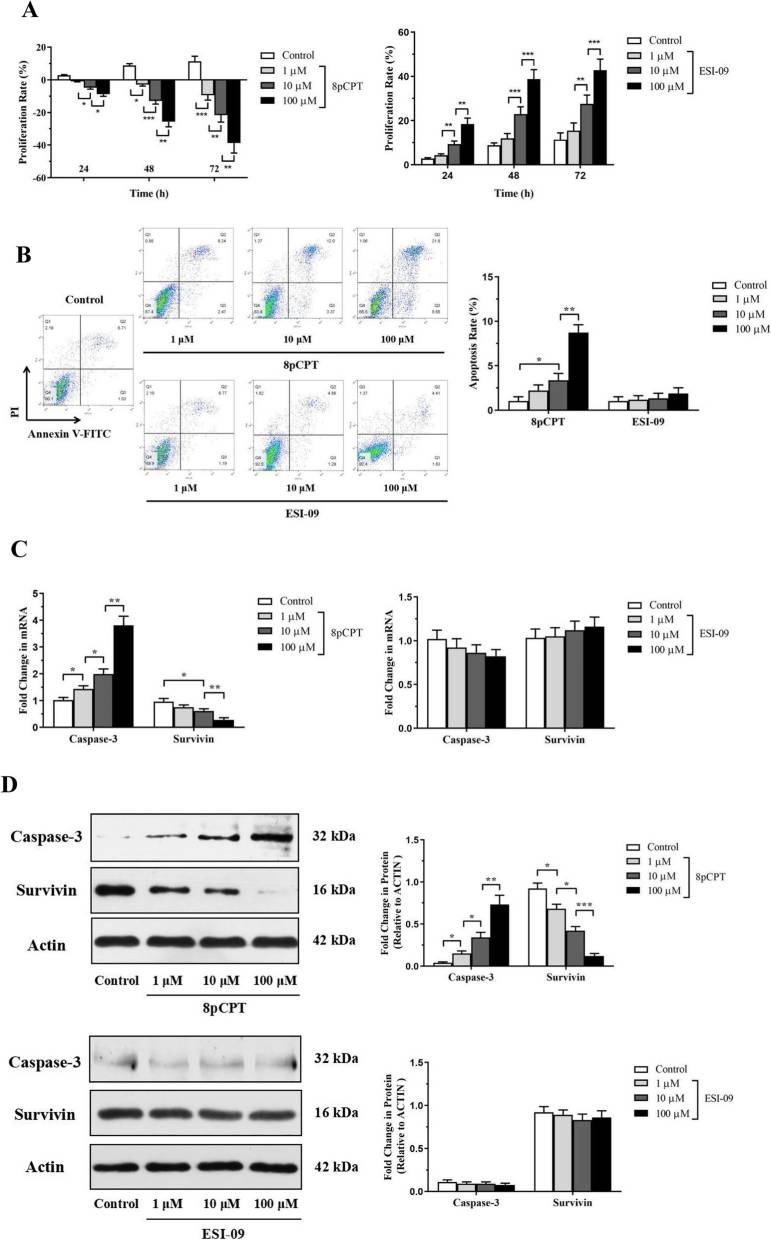 Fig. 2. Effects of Epac regulators on the proliferation and apoptosis of ASMCs (Chen YF, Huang G, et al., 2019).
Fig. 2. Effects of Epac regulators on the proliferation and apoptosis of ASMCs (Chen YF, Huang G, et al., 2019).
Check all containers for leakage or breakage. Directly and immediately transfer the cells from dry ice to liquid nitrogen and keep the cells in liquid nitrogen until they are needed for experiments.
The recommended medium is SuperCult® Mouse Smooth Muscle Cell Medium (cat# CM-2008S), SuperCult® Murine Smooth Muscle Cell Medium (cat# CM-1086X) or SuperCult® Mouse Smooth Muscle Cell Medium Kit (cat# CM-4696L).
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Preferred supplier
I am very impressed with Creative Bioarray's product quality and professionalism, making them my preferred supplier.
13 Feb 2023
Ease of use
After sales services
Value for money
Write your own review