- You are here: Home
- Applications
- 3D Spheroid & Organoid Culture
- Organoid Platform for Drug Development
Applications
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
Organoid Platform for Drug Development
Organoids represent a significant advancement in tissue engineering and regenerative medicine, as they mimic the architecture and functionality of real organs in a laboratory setting. A key feature of organoids is the self-organization of cells, allowing them to develop distinct structures and functions similar to those found in actual organs. This process is influenced by various factors, including the extracellular matrix, biochemical signaling, and environmental conditions. As a result, organoids typically comprise multiple cell types that can communicate and interact in ways analogous to their in vivo counterparts.
These 3D structures are derived from stem cells or pluripotent cells and can be generated from various tissues, including the brain, liver, kidney, intestine, and pancreas, among others. In addition to their research applications, organoids hold potential for personalized medicine, as patient-derived cells can be used to create organoids that reflect individual disease states, enabling tailored therapeutic strategies.
Multiple Applications of Organoids
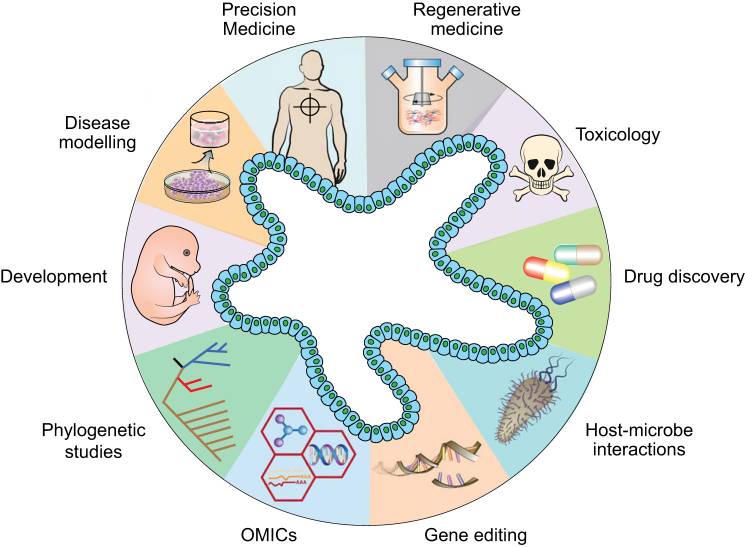 Figure 1. Diverse applications of organoid technology. [1]
Figure 1. Diverse applications of organoid technology. [1]
- Disease Modeling: Organoids derived from tumor tissues enable researchers to study cancer biology, test drug responses, and understand tumor heterogeneity. Additionally, they can model genetic diseases by replicating affected tissues.
- Drug Discovery and Toxicity Testing: Organoid models facilitate high-throughput drug screening and the assessment of drug efficacy and toxicity, offering a more accurate representation of human responses compared to traditional cell lines.
- Personalized Medicine: By creating patient-specific organoids, clinicians can test the effectiveness of various treatment options on an individual's tumor or disease model, leading to more personalized therapeutic strategies.
- Gene Editing: Organoids can be generated from the cells of patients with genetic disorders and edited to correct specific mutations. This process enhances the study of disease mechanisms and aids in the development of potential treatments.
Features of Creative Bioarray's Organoids
- Diverse Indications: Access a range of organoids representing over 20 different organ and tissue types.
- Genetic Perturbation Capabilities: Utilize advanced gene editing techniques, such as RNA interference and CRISPR/Cas, to identify and validate biological targets and discover mechanistic biomarkers.
- Integrated Model Systems: Benefit from complementary in vitro Patient-Derived Xenograft Organoids (PDXOs) and in vivo Patient-Derived Xenografts (PDX) models, which enhance predictive accuracy and facilitate faster, more informed transitions in studies.
- Clinically Relevant Models: Utilize Stem Cells (SCs) harvested directly from patients (Patient-Derived Organoids, PDOs) or from xenograft tissues (Patient-Derived Xenografts, PDXOs). These models effectively mimic the complex architecture of in vivo tissues while allowing for unlimited expansion for large-scale screening.
- Living Biobanks: Access a comprehensive organoid database featuring genomic, transcriptomic, and pharmacological data for advanced research and analysis.
Creative Bioarray presents a state-of-the-art Organoid Platform designed to transform drug development and biomedical research. By leveraging advanced organoid and organoid-immune co-culture models, this platform offers highly accurate and predictive solutions that closely replicate human biology.
- Organoid Models
- Organoid-Immune Co-Culture Models
- Organoid Drug Screening
- Organoid-based High-throughput Screening
Reference
- Corrò, Claudia et al. "A brief history of organoids." American journal of physiology. Cell physiology vol. 319,1 (2020): C151-C165. doi:10.1152/ajpcell.00120.2020
Explore Other Options
For research use only. Not for any other purpose.

