- You are here: Home
- Services
- In Vivo DMPK Services
- PK/PD Biomarker Analysis
Services
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
PK/PD Biomarker Analysis
The therapeutic effects of new candidates are influenced by ADME in the body. PK/PD assays are essential assays for researchers to develop new candidates. In addition to assessing the dynamic properties of new compounds through PK/PD, biomarker analysis is also used to predict drug efficiency in vivo. Nowadays, biomarker analysis is analytical measures that provide information that can be predicted or as an alternative endpoint.
Biomarkers contribute to the understanding of drug action patterns, can be involved in response to therapeutic management assessment, and are directly related to pharmacokinetic (PK) endpoints. They play an important role in drug development stage. At a time when drug approval rates are declining, the pharmaceutical industry needs biomarkers to predict efficacy and safety well. Therefore, we need to use some assays to quantify levels of biomarkers and explore identification assays to support different drug developmental stages.
Biomarker assays are often limited by rare and precious samples. Current immunoassays require pooling of samples from multiple animals and lack dynamic range and sensitivity. Miniaturizing and automating devices on Bioarray's platform can significantly solve these issues.
Our advantages:
- Sensitivity and dynamic range (quantitative limit reduced to unit pg/ml)
- Quick results (automated analysis workflow)
- Low sample consumption (4 μl are enough for duplicate assays)
Creative Bioarray's bioanalytical laboratories can develop and validate methods for analyzing biomarkers in preclinical and clinical samples such as plasma, serum, urine, tissue and cerebrospinal fluid. We provide assays depending on your development phase and purpose regarding the biomarker:
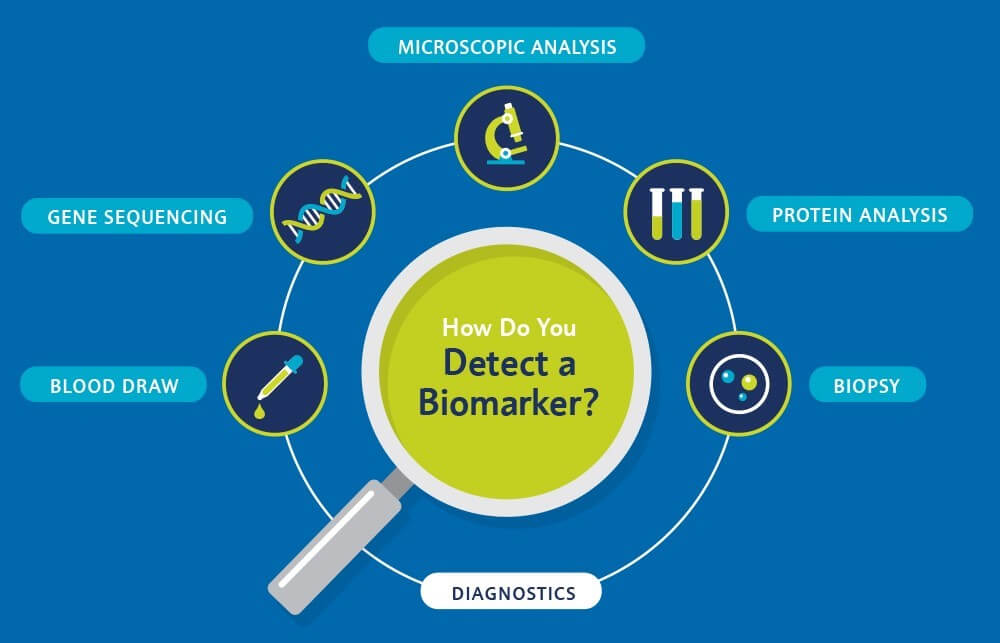
Service include:
- Predict drug response
- Monitor disease
- Prognosis of patients
- Steer dose selection
- Patient stratification
Analysis instrument:
- LC-MS/MS systems coupled with HPLC and UPLC
- Immunohistochemistry platform
- Real Time PCR platform
- ELISA platform
- Luminex xMAP
We ensure reliability through analytically validated methods and guarantee that our clients' development needs are addressed. We can also provide the services and expertise needed to accelerate drug development, and our capabilities include not only biomarker recognition, but also other downstream clinical applications. If you have any special needs or questions regarding our services, please feel free to contact us to get support from our experienced experts. We look forward to working with you in the future.
Explore Other Options
For research use only. Not for any other purpose.

