- You are here: Home
- Services
- Drug Toxicity Services
- Hepatotoxicity
Services
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
Hepatotoxicity
As a heterogeneous organ with many vital functions, liver plays a central role in transforming and clearing chemicals thus is susceptible to the toxicity from these agents. Liver suffers the most from drug toxicity, making drug-induced liver injury (DILI) the leading cause of drug failures. Hepatotoxicity implies chemical-driven liver damage. It can be induced by a drug itself or indirectly by the generation of reactive metabolites.
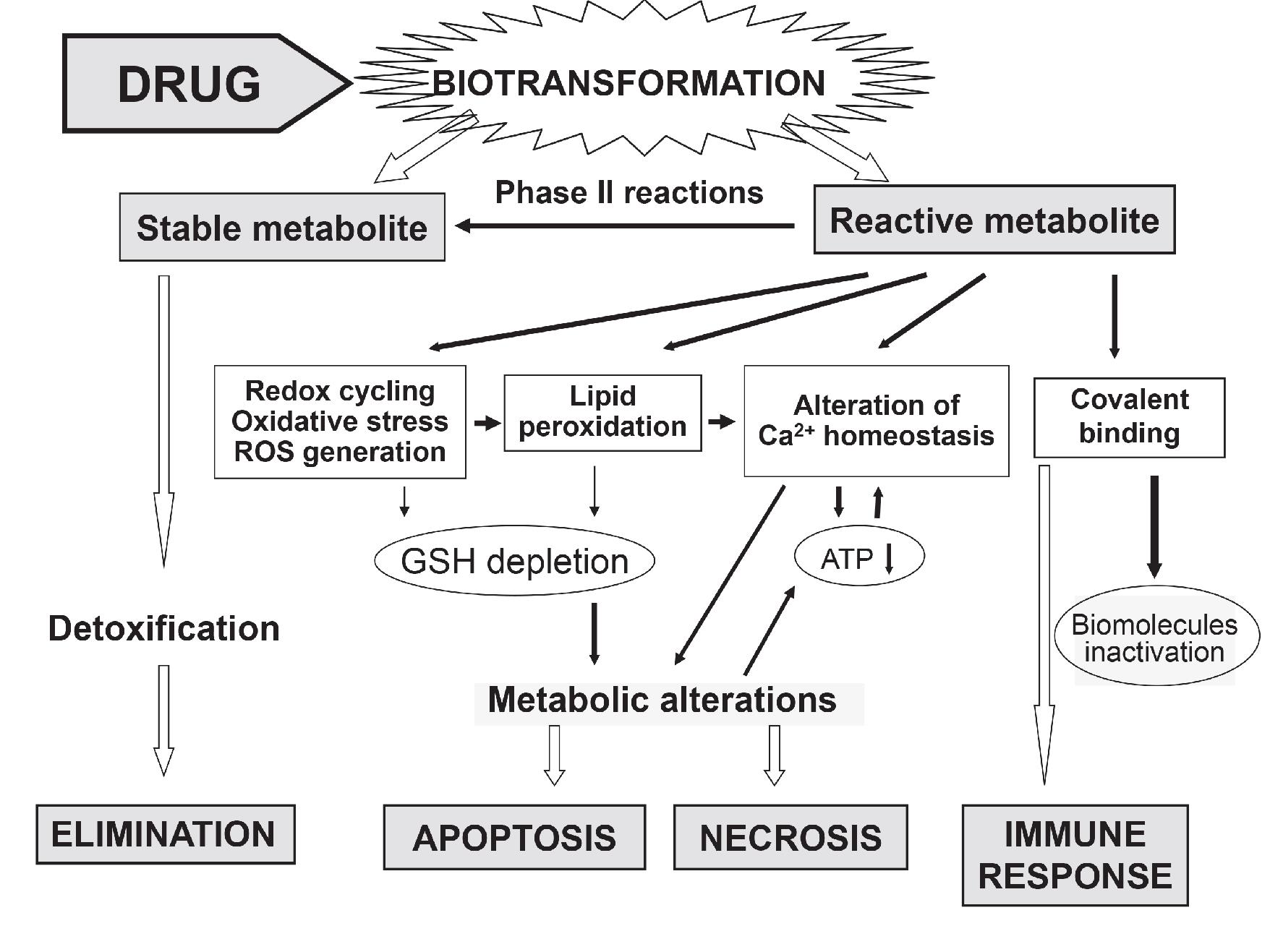 Fig.1 Molecular events leading to drug-induced liver cell damage and death
Fig.1 Molecular events leading to drug-induced liver cell damage and death
The etiology of drug induced liver disease is still under debate but is generally regarded as a continuum between an activated immune response and hepatocyte metabolic dysfunction most often resulting from an intermediate reactive metabolite. In vitro cell-based toxicity assays could mimic in vivo tissue studies and in turn provide a reliable tool for safety evaluation in early stages of drug discovery.
Creative Bioarray provides a series of in vitro models and assays to help researchers assess drug hepatotoxicity potential in preclinical stage.
Your Needs
- Innovative efficacy assays on Hepatology
- Test the safety of your drugs or active molecule products in preclinical researches
- Customized services for special requirements on efficacy or safety tests
Our Capability
Creative Bioarray has developed a multiplexed hepatotoxicity assays using various in vitro hepatology models, such as primary hepatocytes, HepG2 cell lines and 3D hepatic models to predict DILI. These multi-endpoint analyses provide a thorough assessment of hepatotoxicity while supplying detailed mechanistic information underlying the observed toxicity. Our experienced scientists will assist you with meeting your objectives and support your projects.
Our services will help you determine potential hepatotoxicity of test compounds from the following perspectives:
- Cell viability / Proliferation / Apoptosis
- Production of reactive oxygen species (ROS)
- Covalent binding of reactive metabolites to proteins
- Protein synthesis inhibition
- Glutathione level
- ATP / Mitochondrial function
- BSEP (bile salt export pump) transporter inhibition:
- Cholestasis
BSEP, expressed almost exclusively in the liver, is critical for the rate of bile acid secretion and associated with hepatotoxicity.
As bile formation is an important function of the liver, inhibition of drugs on BESP may cause bile salt accumulation in the liver, resulting in DILI.
Accumulation of bile salts can be labelled with fluorescent probes and subsequently measured via high content imaging.
In Vitro Hepatotoxicity Testing Models
- Creative Bioarray provides various 2D culture models including HepG2 cell line, human primary hepatocytes and iPSC-derived hepatocytes for investigation of potential adverse hepatotoxicity.
- Creative Bioarray offers hepatotoxicity screening service using 3D cell culture models derived from hepatocytes of several different species. As a world-class supplier of 3D cell culture models, and with extensive experience in drug discovery through years of practice, Creative Bioarray is able to provide you with highly reliable data on drug hepatotoxicity.
Need Help?
If you have any needs or questions, please contact us. Our customer service representatives are available 24hr a day! We thank you for choosing Creative Bioarray services!
Reference
- Gómez-Lechón MJ; et al. Cell-based models to predict human hepatotoxicity of drugs. Rev. Toxicol. 2014, 31: 149-156.
Explore Other Options
For research use only. Not for any other purpose.

