- You are here: Home
- Applications
- Mucosa
- In vitro Eye Irritation Test
Applications
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
In vitro Eye Irritation Test

The human eye is responsible for the optical perception of the surrounding world. To allow light to reach sensory cells on the retina the eye is protected only by a relatively thin transparent tissue called the cornea. In contrast to human skin, the cornea epithelium is not cornified and is thus more prone to mechanical or chemical injury. Due to the eyes’ vulnerability and their outstanding importance for the individual, standardized test protocols have been developed to assess the potential hazard exerted by chemical substances and consumer products on the human eye.
The traditional standard assays, such as the Draize eye test, predict the effect on the human eye by the reactions observed in different animal models. However, due to ethical concerns, scientific reasons and a change in international legislation there is an increasing demand to replace the in vivo methods by alternative in vitro eye irritation test, which involves in using ex vivo and in vitro models.
With over 10 years’ experience of working on cell & tissue products and services, Creative Bioarray provides in vitro reconstructed human cornea-like Epithelium (RhCE) model and in vitro eye irritation test services to help industries evaluate eye irritation potential for their chemicals and cosmetic products.
Your Needs
- You are looking for RhCE model for your researches?
- You wish to evaluate the eye irritation potential for your ingredients, products or chemicals?
- You’d like to find a customized service of in vitro eye irritation test?
Our Capability
In vitro 3D models available
- Reconstructed human cornea-like Epithelium (RhCE) model
- RhCE model is a highly predictive non-animal alternative to assess eye irritation, drug delivery, infection, wound healing/tissue regeneration and disease modeling.
- Ex vivo human cornea model
- Human corneal epithelial cells (HCECs)
- HCE-T cell line
Assays available
In vitro eye irritation test service based on OECD TG 492.
Assay Examples
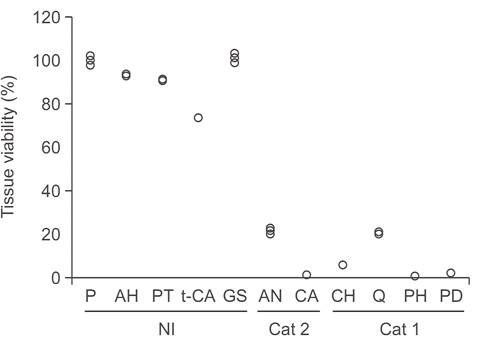 Fig.1. Tissue viability obtained with the optimized eye irritation protocol by using RhCE model. Tissue viability results for 11 reference substances.
Fig.1. Tissue viability obtained with the optimized eye irritation protocol by using RhCE model. Tissue viability results for 11 reference substances.
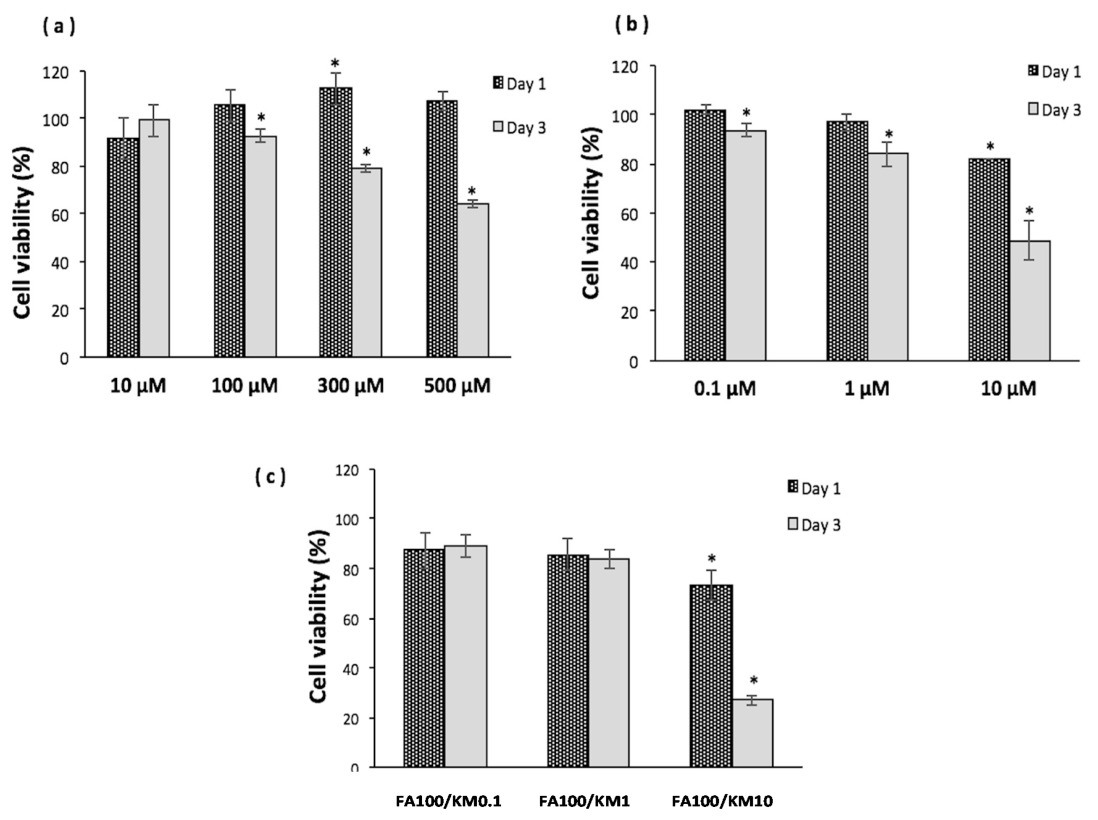 Fig.2. Cell viability of human corneal epithelial cells (HCECs) after incubation with varying concentrations of (a) ferulic acid (FA), (b) kaempferol (KM) and (c) a FA/KM mixture for one or three days.
Fig.2. Cell viability of human corneal epithelial cells (HCECs) after incubation with varying concentrations of (a) ferulic acid (FA), (b) kaempferol (KM) and (c) a FA/KM mixture for one or three days.
Related Products and Services
Choose our models to perform screening assays in house, or choose our assays and services directly!!
- Reconstructed human cornea-like Epithelium (RhCE) model
- Ex vivo human cornea model
- In vitro eye irritation test services
Our customer service representatives are available 24hr a day! We thank you for choosing Creative Bioarray services!
Explore Other Options
For research use only. Not for any other purpose.

