Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

Gallbladder Tumor Cells
- Product List
- Background
- Applications
- Scientific Data
- FAQ
The gallbladder, a small pear-shaped organ located just beneath the liver, is responsible for storing and concentrating bile, a crucial fluid that aids in the digestion of fats. However, in some individuals, abnormal cell growth can occur within the gallbladder, leading to the formation of tumors. These tumors can vary in their characteristics, ranging from benign (non-cancerous) to malignant (cancerous), each with its own set of implications for the patient's health and well-being.
Molecular Characteristics of Gallbladder Tumor Cells
- Genetic mutations and alterations. Mutations in the TP53 gene are commonly observed in gallbladder tumor cells, contributing to uncontrolled cell proliferation, evasion of apoptosis, and tumor progression. Activating mutations in the KRAS gene are prevalent in gallbladder cancer, driving aberrant signaling pathways involved in cell survival, proliferation, and invasion. Mutations in the PIK3CA gene lead to dysregulated PI3K/AKT/mTOR signaling cascades in gallbladder tumor cells, promoting cell growth and survival. Targeting these mutations and alterations represents a potential therapeutic strategy.
- Epigenetic alterations. Aberrant DNA methylation patterns play a crucial role in gallbladder cancer pathogenesis, silencing tumor suppressor genes and activating oncogenes. Alterations in histone modifications influence gene expression patterns in gallbladder tumor cells, regulating chromatin structure and transcriptional activity. Altogether, epigenetic modifications contribute to tumor heterogeneity and may serve as diagnostic and prognostic markers.
- Signaling pathway dysregulation. Dysregulated Wnt/β-catenin signaling is frequently observed in gallbladder cancer, promoting cell migration, invasion, and epithelial-mesenchymal transition. Inhibiting aberrant Wnt pathway activation may impede tumor progression. Aberrant Notch signaling in gallbladder tumor cells influences cell fate decisions, proliferation, and apoptosis, contributing to tumor growth and metastasis. Targeting Notch signaling components presents a promising avenue for therapeutic intervention.
Cancer research and drug development
Gallbladder tumor cells have become an invaluable tool in the field of cancer research, serving as a model system to explore the genetic and molecular mechanisms that drive the initiation and progression of this disease. By studying the behavior and characteristics of these cells, researchers have been able to identify novel therapeutic targets and develop innovative drug candidates that hold promise in the treatment of not only gallbladder cancer but also other types of cancers.
Tissue engineering and regenerative medicine
By harnessing the proliferative and adaptable nature of gallbladder tumor cells, researchers have been able to create complex tissue structures that mimic the characteristics of the native gallbladder. These models can be employed for drug testing, toxicology studies, and the development of personalized therapeutic strategies, ultimately accelerating the translation of novel treatments from the laboratory to the clinic.
Furthermore, scientists have also investigated the potential of gallbladder tumor cells in the field of regenerative medicine. By understanding the signaling pathways and cellular mechanisms that drive the growth and differentiation of these cells, researchers aim to explore their ability to contribute to the repair and regeneration of damaged or diseased tissues, opening up new avenues for the treatment of various medical conditions.
PLEK2 Promotes Migration, Invasion, and Metastasis of GBC Cells
Gallbladder cancer (GBC) is an extremely malignant tumor with a high mortality rate. Little is known about its invasion and metastasis mechanism so far. To investigate the causal role of PLEK2 in GBC progression, PLEK2 down-regulation NOZ and GBC-SD cells (NOZ-shPLEK2, GBC-SD-shPLEK2, respectively), as PLEK2 overexpression NOZ and GBC-SD cells (NOZ-PLEK2, GBC-SD-PLEK2, respectively) were constructed.
Cell proliferation assay showed no difference between PLEK2 knockdown and control cells. Meanwhile, the transwell migration assay indicated that PLEK2 knockdown or overexpression significantly inhibited or promoted cell migration in corresponding GBC cells, respectively. Similar to the migration assay, the transwell invasion assay also showed the same results (Fig. 1a, c). Therefore, these in vitro studies indicated that PLEK2 promoted GBC cell migration and invasion. In addition, whether PLEK2 could promote GBC tumor metastasis in xenograft models was investigated. In vivo studies showed that PLEK2 knockdown exhibited fewer liver metastatic foci whereas PLEK2 overexpression displayed more liver metastatic foci compared to the control group (Fig. 1b, d).
Given some previous studies have shown the involvement of PLEK2 in actin remodeling, the morphological change was detected following PLEK2 knockdown or overexpression. Consistent with previous studies, PLEK2 knockdown cells displayed small and round shapes whereas PLEK2 overexpression cells exhibited spindle-like shapes compared to control cells (Fig. 1e). The change in the cell morphology might facilitate their motility. As the EMT process plays an indispensable role in tumor metastasis, whether the function of PLEK2 in cell spreading promoted the EMT process was investigated. As shown in Fig. 1f, PLEK2 knockdown suppressed Fibronectin and N-cadherin, whereas enhanced E-cadherin expression. On the contrary, PLEK2 overexpression enhanced Fibronectin and N-cadherin, whereas suppressed E-cadherin expression.
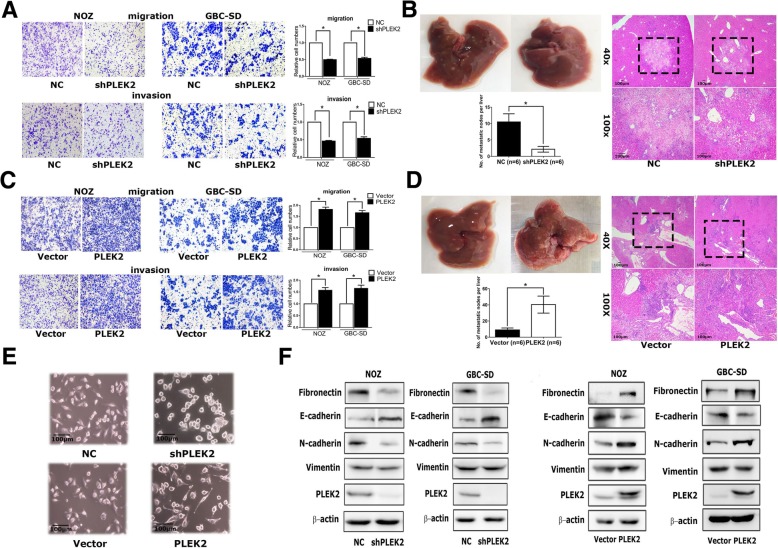 Fig. 1 Effects of PLEK2 on the migration, invasion, and metastasis of GBC cells. (Shen H, et al, 2019)
Fig. 1 Effects of PLEK2 on the migration, invasion, and metastasis of GBC cells. (Shen H, et al, 2019)
Silencing ARAF Inhibits GBC Cell Proliferation, Migration and Invasion
ARAF is a member of the RAF kinase family that is necessary for mitogen-activated protein kinase (MAPK) activation in various malignancies, including lung, colorectal, pancreatic, and breast cancers. As the most common biliary tract tumor, gallbladder cancer (GBC) seriously harms human health while the function of ARAF in GBC remains elusive.
ARAF knockdown was employed to study its function in GBC cells. ARAF expression was examined in GBC-SD and SGC-996 cells transfected with ARAF siRNA or siRNA control. After siRNA transfection, ARAF mRNA and protein levels were both significantly lower than controls (Fig. 2a and 2b). It was demonstrated that the ARAF siRNA successfully silenced endogenous ARAF in GBC cells. Inhibiting the rapid growth of cancer cells is an important way to treat cancers. As shown in Fig. 2c, the CCK-8 assays showed that ARAF siRNA inhibited the proliferation of different GBC cell lines, including GBC-SD cells and SGC-996 cells. Interestingly, PCNA, a reliable indicator of cell proliferation, was also higher in the control siRNA group compared with ARAF knockdown (Fig. 2d). Furthermore, colony formation was significantly reduced in the ARAF siRNA group, compared with controls (Fig. 2e and 2f).
The role of ARAF on the migration and invasion of GBC cells was further explored by wound healing and transwell assays. As shown in Fig. 3a and 3b, ARAF knockdown significantly attenuated cell migration compared with controls. The invasiveness of SGC-996 cells is too poor to use for transwell assays, GBC-SD cells were employed to investigate the role of ARAF on cell invasion. The invasion of GBC-SD cells was remarkably suppressed after ARAF was knocked down (Fig. 3c). These results indicate that silencing ARAF inhibits the migration and invasion of GBC cells.
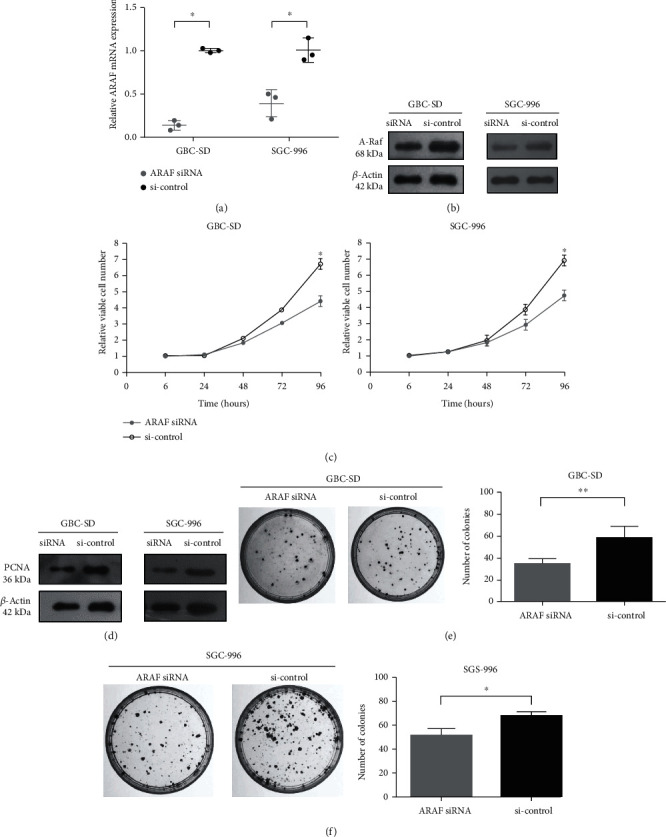 Fig. 2 Downregulated expression of ARAF inhibits GBC cell proliferation. (Lin W, et al., 2020)
Fig. 2 Downregulated expression of ARAF inhibits GBC cell proliferation. (Lin W, et al., 2020)
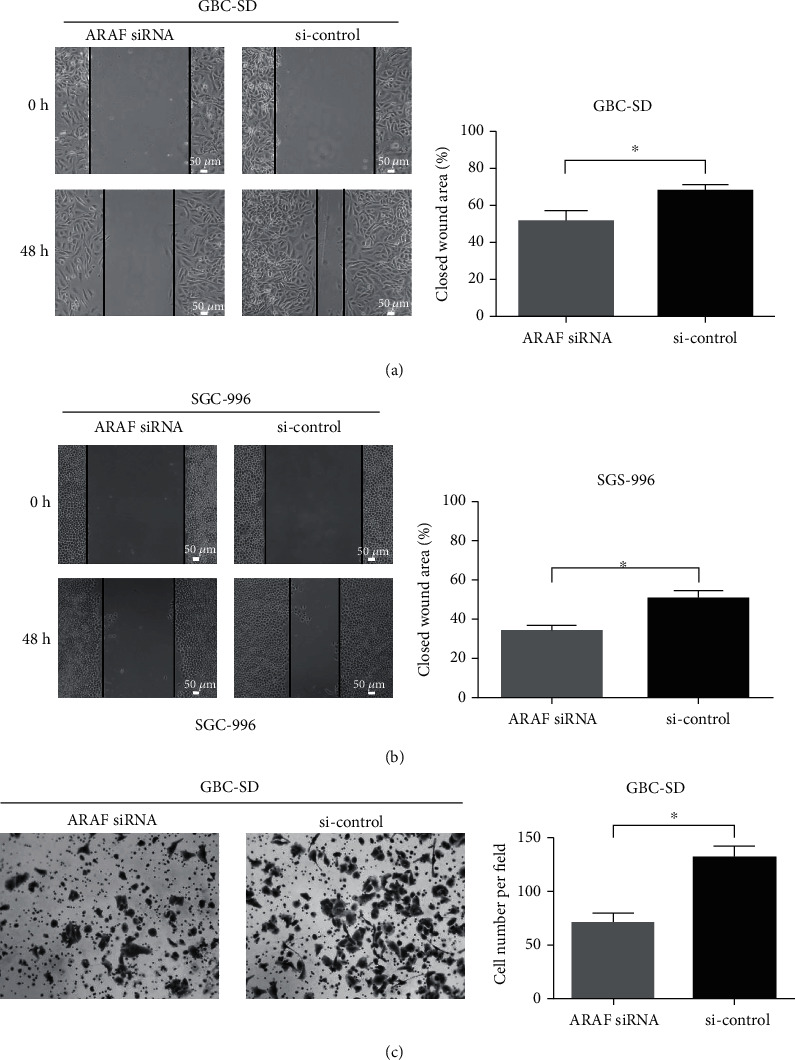 Fig. 3 ARAF promotes migration and invasion of GBC cells. (Lin W, et al., 2020)
Fig. 3 ARAF promotes migration and invasion of GBC cells. (Lin W, et al., 2020)
Gallbladder tumors can be classified into various types, including adenocarcinomas (the most common), squamous cell carcinomas, neuroendocrine tumors, and adenosquamous carcinomas.
Research on gallbladder tumor cells can lead to the discovery of novel therapeutic targets, biomarkers for early detection and prognosis, personalized treatment approaches, and a deeper understanding of the molecular mechanisms driving tumor development and progression.
Gallbladder tumor cells often exhibit altered metabolic profiles, including increased glycolysis, glutamine metabolism, and lipid synthesis. These metabolic adaptations allow gallbladder tumor cells to support their rapid proliferation and survive in the tumor microenvironment.
Description: Japanese gallbladder carcinoma established from metastated ascite.
Description: Japanese gallbladder tumor passed through a nude mouse.
Description: Japanese gallbladder carcinoma from the same patient of TGBC1TKB.
Description: Japanese gallbadder carcinoma metastated to lymph node from the same patient of TGBC2TKB.
Description: Human poorly differentiated cholangiocarcinoma cell line established from biliary tract.
Description: Human mixed (papillary and non-papillary) cholangiocarcinoma cell line established from biliary tract.
Description: Human cell line derived from cholangiocarcinoma.

