- You are here: Home
- Applications
- Skin
- Dermatology
- Skin Sensitization
Applications
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
Skin Sensitization

Skin sensitization is defined as allergic response to a substance after skin contact. A skin sensitiser refers to a substance that will lead to an allergic response following skin contact as defined by the United Nations Globally Harmonized System of Classification and Labelling of Chemicals.
The assessment of skin sensitization has typically involved the use of laboratory animals. The classical method is using guinea-pigs. While, more recently mechanistically-based in chemico (OECD TG 442C) and in vitro (OECD TG 442D) test methods have been adopted for contributing to the evaluation of the skin sensitization hazard potential of chemicals as a valid animal test alternative.
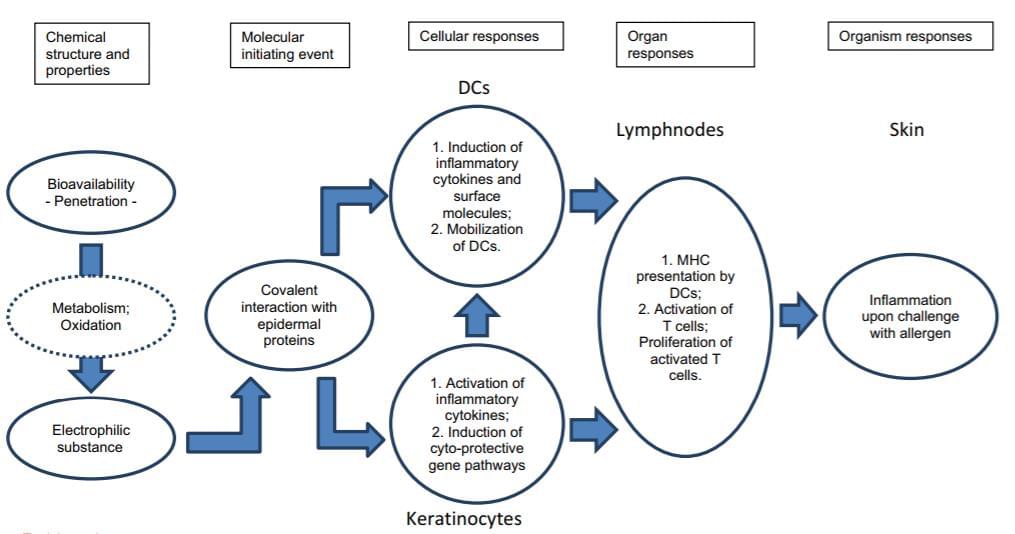 Fig 1. OECD flow diagram of the pathways associated with skin sensitization. The Adverse Outcome Pathway (AOP) framework provides a structure for collecting, organizing and evaluating the available data that describe the compound and the events resulting in an adverse outcome at a biological level of organization.
Fig 1. OECD flow diagram of the pathways associated with skin sensitization. The Adverse Outcome Pathway (AOP) framework provides a structure for collecting, organizing and evaluating the available data that describe the compound and the events resulting in an adverse outcome at a biological level of organization.
Creative Bioarray team provides in vitro skin models and in vitro skin sensitization assays in order to support pharmaceutical and cosmetic companies for their drug or product development related to skin sensitization.
Your Needs
- You are looking for an in vitro skin model to evaluate a skin sensitiser?
- You wish to evaluate chemicals, ingredients or formulations of drugs or products for their skin sensitization hazard?
- You'd like to find a customized in vitro testing service related to skin sensitization?
Our Capability
In vitro skin models available
Assays available
Creative Bioarray can provide a range of services to evaluate skin sensitization by using our skin models or cell lines:
- The In Vitro Sensitization Assay (IVSA) is a keratinocyte activation test. IVSA uses an ELISA method to measure IL-18 release from a topically treated reconstructed 3D human keratinocyte tissue model.
- The Human Cell Line Activation Test (h-CLAT) is a dendritic cell activation test. The h-CLAT uses flow cytometry to measure CD86 and CD54 expression on treated THP-1 Cell line in culture, based on OECD TG Draft (May 2015).
- The Direct Peptide Reactivity Assay (DPRA) is an in chemico method used to predict epidermal protein binding. Binding of epidermal proteins is the molecular initiating event on the AOP. The DPRA uses HPLC to measure the depletion of synthetic peptides in solution following exposure to test chemicals, based on OECD TG 442C (February 2015).
- The KeratinoSens Assay is a cell-based reporter gene assay which identifies skin sensitizers by measuring the induction of luciferase under the control of the antioxidant response element (ARE) derived from the human AKR1C2 gene. In the proposed Adverse Outcome Pathway (AOP) leading to skin sensitization, this method addresses the second key event, gene expression in keratinocytes associated with the antioxidant/electrophile response element (ARE)-dependent pathway. The assay was endorsed by ECVAM, and the OECD followed with a test guideline (OECD 442D).
Study Example
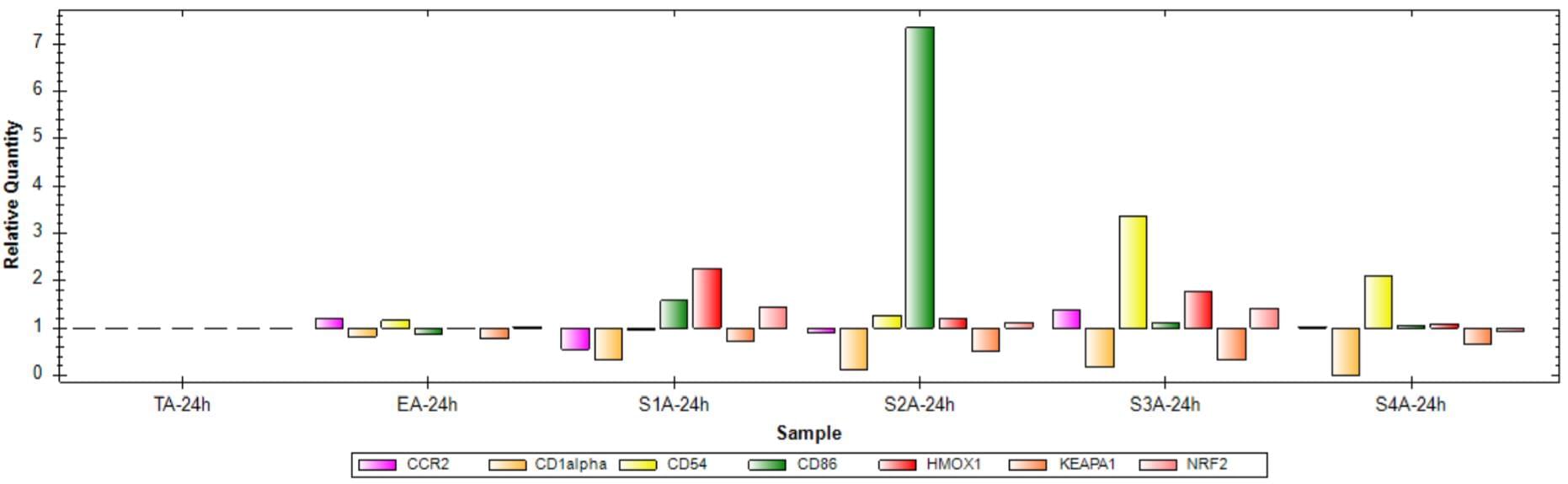 Fig 2. Gene expression analysis after treatment for 24 hours with sensitizers
Fig 2. Gene expression analysis after treatment for 24 hours with sensitizers
Related Products and Services
Choose our models to perform screening assays in house, or choose our assays and services directly!!
- Full thickness skin model (FTSK)
- Reconstructed human epidermis model (RHE)
- THP-1 Cell line
- Skin sensitization test services
Our customer service representatives are available 24hr a day! We thank you for choosing Creative Bioarray services!
In vitro Skin Models:
Explore Other Options
For research use only. Not for any other purpose.

