- You are here: Home
- Services
- Histology Services
- Transmission Electron Microscopy
- Single-Particle Cryo-EM
Services
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
Single-Particle Cryo-EM
Single-Particle cryo-EM is an electron microscopy method used to determine structures of individual proteins and other macromolecular complexes. Structures of macromolecules can be determined from cryo-EM images at near-atomic and sometimes true atomic resolution.
Single-Particle cryo-EM can be used to answer specific questions concerning protein-protein interactions and identify protein/domain assembly. Antibodies or Fab can be visualized when bound to their antigen. Besides, the combination of cryo-EM and AI provides an opportunity to be the new direction of future development of cryo-EM. The rapid development of cryo-EM will make it as an indispensable part of modern drug discovery.
Creative Bioarray offers Single-Particle Cryo-EM services, including cryo-EM SPA sample preparation, EM imaging, data processing and interpretation.
Advantages of Single-Particle Cryo-EM
- High Resolution: Single-Particle Cryo-EM offers high-resolution imaging which can often reach near-atomic resolution. This feature allows detailed studies of biological structures or even identification of chemical compounds bound to the structure.
- Structure Determination of Complex Systems: This technology is capable of determining the structures of complex systems, such as membrane proteins, large protein assemblies and viruses, which are difficult to study using other methods.
- No Crystallization Needed: Single-Particle Cryo-EM does not require crystallization of the sample. This is a significant advantage as many biological samples are difficult or impossible to crystallize.
- Heterogeneous Sample Analysis: It allows analysis of heterogeneous samples and can provide insights into multiple conformational states of a protein complex in one experiment.
- Direct Image of Solution State: It provides a direct image of the protein complexes in their near-native state, preserving their functional and biologically relevant forms.
- Minimal Sample Amount: Compared to other structure determination methods, Single-Particle Cryo-EM requires much less amount of the sample.
- Speed: It's faster than some other techniques in determining the 3D structure of proteins, complexes, or assemblies.
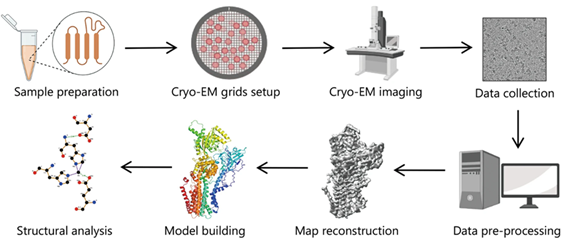 Figure 1. Typical workflow of single particle cryo-EM for structural analysis (Zhu, Kong-Fu, et al. "Applications and prospects of cryo-EM in drug discovery." Military Medical Research 10.1 (2023): 10.
Figure 1. Typical workflow of single particle cryo-EM for structural analysis (Zhu, Kong-Fu, et al. "Applications and prospects of cryo-EM in drug discovery." Military Medical Research 10.1 (2023): 10.
Workflow of Single-Particle Cryo-EM
- Purify: Single-particle cryo-EM requires purification and structural integrity of the specimen for accurate 3D reconstructions. It necessitates maintaining the specimen in a buffer solution for biochemical activity. The molecule concentration should be high for microscopic study, while avoiding aggregation. Meanwhile, uniform molecular conformation should be ensured through optimized experimental conditions.
- Plunge freeze: The specimen is initially frozen to avoid freeze-drying within the microscope's vacuum. Rapid freezing helps prevent the disruption of specimen structure through ice crystal formation. Firstly, the specimen solution is applied to a TEM grid. Excess liquid is then blotted away briefly before the grid is submerged in liquid ethane or an ethane/propane mix. This swift wetting and cooling process forms non-crystalline or vitreous ice around the specimen. The image displays the Cryoplunge® 3 system just before blotting and specimen submersion into the liquid cryogen.
- Transfer to TEM: The frozen specimen is transitioned into a specific TEM holder that sustains it at liquid nitrogen temperature to avoid contamination. The specimen is safeguarded in a cryo-workstation during loading and encased in a cryo-shield when transferring to the TEM. The image illustrates the withdrawal of the cryo-transfer holder from the workstation, ready for TEM insertion.
- Image specimen: Specimens are vulnerable to structural damage when exposed to electrons, limiting the total dose to 10 - 30 e-/Å2 before losing high-resolution information. To mitigate this, low-dose methods are used for location and focus before image capture. Gatan's direct detector electron counting and super-resolution modes deliver high-quality, high signal-to-noise ratio images of delicate biological samples, revealing water molecules, ions, and ligand structures in the 3D particle reconstruction. Image quality can be further enhanced by utilizing the dose fractionation feature on Gatan's cameras, which captures full frames at up to 75 frames per second to later correct for specimen motion and reduce drift.
- Analyze and reconstruct: The software aids in data analysis and conversion into various formats after imaging. The data from the cameras can be incorporated into numerous third-party software tools for 3D reconstruction and visualization, such as EMAN, Frealign, Relion, etc. The displayed image is a 3D cryo-EM density of a 2.8 Å resolution 20S proteasome.
Quotation and ordering
Our customer service representatives are available 24hr a day! We thank you for considering Creative Bioarray as your Single-Particle Cryo-EM partner.
Explore Other Options
For research use only. Not for any other purpose.

