- You are here: Home
- Services
- In Vitro DMPK Services
- In Vitro Metabolism Services
- S9 Stability Assay
Services
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
S9 Stability Assay
With a highly automated approach, Creative Bioarray provides a highly cost-efficient S9 stability assay as one of our in vitro metabolism services and delivers consistent and high-quality data.
S9 Stability Assay Introduction
- Hepatic in vitro metabolism studies
- Drug detoxification and drug metabolism are primarily carried out in the liver. Following oral administration, drugs are absorbed and transported to the liver to be metabolized primarily in this organ. About three-quarters of the drugs cleared via metabolism are metabolized by hepatic cytochrome P450 (CYP)-mediated metabolism.
- Hepatic in vitro metabolism studies using hepatocytes, 9000g supernatant (S9), and microsomal fractions can give out valuable preliminary information on the in vitro metabolism of xenobiotics and shed light on the in vivo metabolism process of a candidate drug by the liver.
- What are the benefits of using S9 stability assay?
- Compared to Hepatocytes which contain the full range of hepatic drug-metabolizing enzymes and serve as the gold standard for metabolism studies, the S9 fraction is not only easier to handle but also sustains the major part of both phase I and phase II drug-metabolizing enzymes, making it one of the perfect alternatives for in vitro drug metabolism studies (Plant, 2004).
- Compared with other drug metabolism systems (such as microsomes and hepatocytes), the metabolism in S9 is often much less. S9 incubation is usually used for qualitative purposes to determine whether metabolites are formed by cytosolic enzymes.
Brief Protocol
- This assay is typically carried out in mouse, rat, and human S9 fraction or microsomes (tests in S9 or microsomes from other species or hepatocytes are available upon request).
- Test compounds are incubated with S9 fraction supplemented with co-factors at 37°C. 4 sampling time-points (0, 15, 30, and 60 minutes) are set up in duplicates.
- At each time point, the reactions are terminated, and samples are centrifuged, filtered, and subjected to LC-MS/MS analysis to evaluate the parent compound concentration.
- This service also includes incubation of 1-2 control drugs and a control reaction without co-factors.
| Test Compound Concentration | 1-3μM (different concentrations available) |
| S9 Concentration | 1-2 mg/mL (different concentrations available) |
| Time Points | 0,15,30,60 minutes (customizable) |
| Controls | Blank control |
| Negative control without co-factors | |
| Positive control: compounds with known activity | |
| Species | Human, rat, mouse, dog, monkey (other species on request) |
| Analysis Method | LC-MS/MS |
| Deliverables | Half life (T1/2) |
| Intrinsic Clearance (CLint) |
Case Study
- Method Validation
Mixed test compound solution and 1 mg/mL S9 solution, then dispensed to the assay plates designated for different time points. After the incubation, the supernatant from each well was transferred into a 96-well sample plate for LC/MS analysis. All tests had three replicates per compound and were validated by the inclusion of a positive control chemical with known stability under the assay conditions, to check for metabolic competency of the test system. Data provided include a percent remaining compound vs. time diagram, disappearance half-life, and intrinsic clearance.
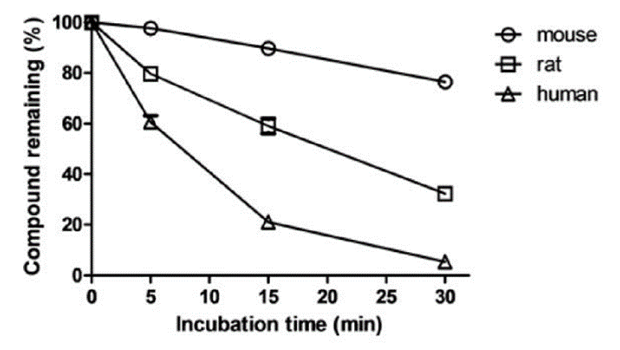 Figure 1. Sample data for the time-dependent depletion.
Figure 1. Sample data for the time-dependent depletion.
- Data analysis
The ln peak area ratio (compound peak area/internal standard peak area) is plotted against time, and the gradient of the straight line is determined.
Half life (T1/2) = 0.693 / K, where K is the rate constant from a plot of ln [concentration] vs. incubation time. |
Intrinsic Clearance (CLint) = 0.693 / T1/2 / mg protein per mL |
Quotation and Ordering
Creative Bioarray's S9 stability assay can be further extended to metabolite profiling and give out more information about the metabolism of your compound. Liver microsomal stability assay and hepatocyte stability assay are also available at Creative Bioarray. Liver S9 fraction (Cat. CSC-C4093X) as a product can be used in genotoxicity tests such as Ames test as a metabolic activator. To find out more about our services and products, please feel free to leave a message below, or contact us.
References
- Plant, Nick. "Strategies for using in vitro screens in drug metabolism." Drug discovery today 9.7 (2004): 328-336.
- Wienkers, Larry C., and Timothy G. Heath. "Predicting in vivo drug interactions from in vitro drug discovery data." Nature reviews Drug discovery 4.10 (2005): 825-833.
Explore Other Options
For research use only. Not for any other purpose.

