Hepatocyte Differentiation Service from iPSC
iPSC-derived hepatocytes provide a stable, readily available cell source for applications previously requiring human primary hepatocyte or hepatoma cell lines, including the investigation of defects in lipid metabolism, protein accumulation, mitochondrial defects, and toxicity screening.
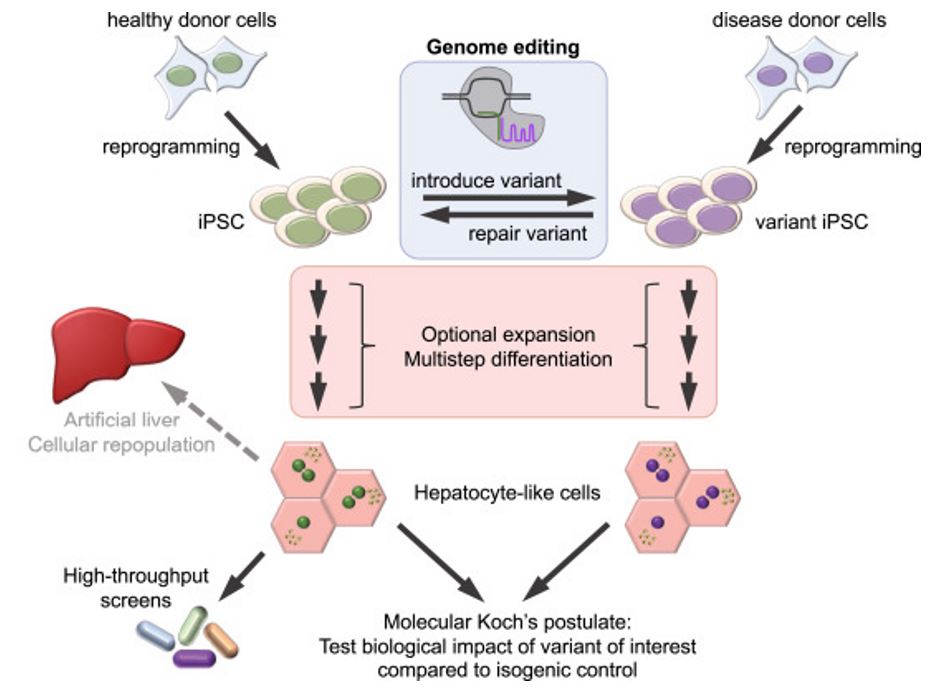 Figure 1. iPSC-derived hepatocytes: a versatile tool in lipid metabolism.
Figure 1. iPSC-derived hepatocytes: a versatile tool in lipid metabolism.
Our Hepatocyte Differentiation Service includes:
- Expansion of a host-derived iPSCs
- Progenitor cell differentiation
- Characterization of progenitor cells
- Terminal differentiation
- Characterization of Progenitor Cells/ Differentiated Cells by ICC (per marker)
Requirements: 5 vials (2.5 million cells per vial) of hiPS or hES cells
Deliverables:
- 30 vials of hepatocytes (12 million viable cells per vial).
- Project Report
Turnaround time: 10-16 weeks
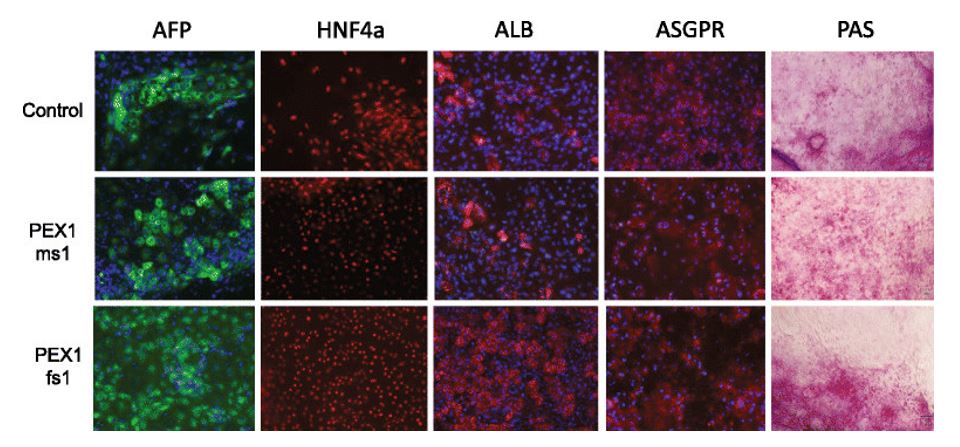 Figure 2. Characterization of iPSC-derived hepatocytes by immunostaining and Periodic acid-Schiff staining (PAS). Expression of AFP (green), HNF4a (red), ALB (red), and ASGPR (red) were detected by immunostaining. Blue represents DAPI staining. PAS staining was used to indicate glycogen storage.
Figure 2. Characterization of iPSC-derived hepatocytes by immunostaining and Periodic acid-Schiff staining (PAS). Expression of AFP (green), HNF4a (red), ALB (red), and ASGPR (red) were detected by immunostaining. Blue represents DAPI staining. PAS staining was used to indicate glycogen storage.
Creative Bioarray is an experienced and outstanding provider of Hepatocyte Differentiation service and differentiation kit. We are dedicated to providing quality data and comprehensive service for your scientific research, and we are pleased to use our extensive experience and advanced platform to offer the best service to satisfy each demand from our customers.
If you have any special need in Hepatocyte Differentiation service, do not hesitate to contact us for this special service. Please let us know what you need and we will accommodate you. We look forward to working with you in the future.
Applications of iPSC-Derived Hepatocytes (iHCs)
Studies in the field of hepatology is limited by the incomplete recapitulation of all major aspects of human hepatic metabolism in most established models. This restricts our ability to study the molecular mechanisms underlying hepatic diseases, and leads to insufficient evaluation of toxicology during drug development, resulting in tremendous unnecessary costs for the pharma industry.
Animal models differ in their metabolism compared to the human system, while primary human cells dedifferentiate rapidly and are not suitable for long-term culture. To overcome these obstacles, several protocols for in vitro differentiation of pluripotent stem cells into hepatocytes have been established. These cells are currently used for modeling inherited and acquired diseases, and to test for drug efficacy and toxicity.
Disease modeling
Disease modeling is an attractive approach to understand the molecular pathway involved in the pathogenesis of liver diseases. Animal models of disease still represent the best way for biomedical research, but the possibility to use patient-derived-iPSC organoids could improve our knowledge regarding the mechanisms that lead to the unique disease phenotype in humans. Moreover, after the discovery of an "easy-to-use" gene editing platform as CRISPR/Cas9, it is possible to introduce or revert specific mutations to replicate the physiopathology in liver organoids.
Metabolic studies
The most common application of iHCs for in vitro drug metabolism and pharmacokinetics (DMPK) studies is to determine the potential of drug-drug interaction (DDI) by inducing gene expression of various CYP450 enzymes. The amount and activity of these enzymes are known to increase when cells are exposed to certain chemical substances. However, an increase in the activity of a particular P450 enzyme might also affect a different drug in a patient's regime, leading to a DDI that could impact the efficacy and safety of more than one drug. Regulatory agencies require an analysis of the DDI potential in hepatocytes for CYP3A4, CYP1A2, and CYP2B6, which indicates whether a clinical DDI study is required.
Toxicology screening
Hepatotoxicity is registered as one of the major causes of termination of drug development process. At present, primary human hepatocytes (pHHs) are the most widely used in vitro model for evaluating hepatotoxicity, however, the availability of pHHs is low since they are isolated from patients undergoing liver transplantation. Hence, hepatocytes derived from human induced pluripotent stem cells (hiPSCs) are of major interest for scientists as an alternative to pHHs for toxicity testing and drug screening due to their capability of self-renewal. It is the hope that iHCs will help to shed new light on pharmacological and toxicological studies due to their genetic and environmental diversity and the ability to establish stable lines from both healthy and diseased individuals as well as the possibility of gene editing makes them an attractive alternative to pHHs.
Scientific Data
In vitro hepatocyte differentiation
A large number of hepatocyte differentiation protocols have been developed during the recent years. Most of them are based on one of four distinct core protocols A-D (Fig. 1) with variation in cytokine and small molecule combination, concentration, and treatment duration.
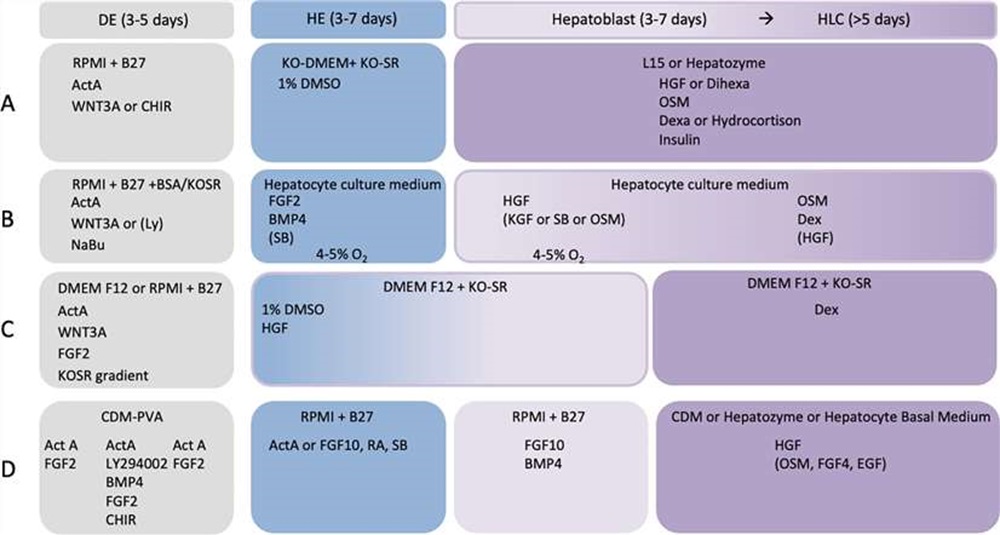 Fig. 1. Basic elements of the four most common hepatocyte differentiation protocols (Nina Graffmann et al., 2022).
Fig. 1. Basic elements of the four most common hepatocyte differentiation protocols (Nina Graffmann et al., 2022).
Differentiation of iPSCs to hepatocytes through a definitive endoderm (DE) stage
Hepatocytes was produced from human iPSCs using a three-step protocol (Fig. 2A). During the differentiation process, the cells underwent morphological changes: iPSCs gradually lost their typical round and dense morphology and after migration formed spiky-shaped DE cells. During the second week of differentiation, at the hepatoblast stage, cells gradually increased in size and, as they matured during the third week, formed polygonal iHCs with distinct canaliculated borders (Fig. 2B). Consistent with the morphological changes observed during the differentiation process (Fig. 2B), immunocytochemical staining further confirmed the expression of hepatic proteins at day 20 of differentiation (Fig. 2C, D). Low-density lipoprotein receptor (LDL-R), asialoglycoprotein receptor (ASGR), alpha fetoprotein (AFP) as well as albumin (ALB) were all expressed in iHCs (Fig. 2C, D).
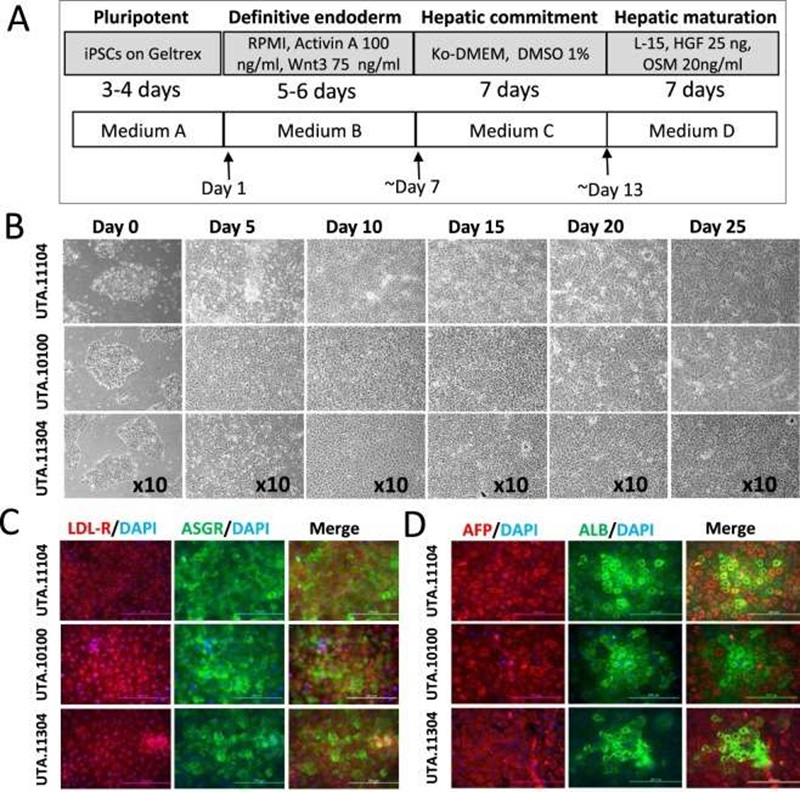 Fig. 2. Hepatic differentiation of iPSCs through the DE stage (Mostafa Kiamehr, et al., 2017).
Fig. 2. Hepatic differentiation of iPSCs through the DE stage (Mostafa Kiamehr, et al., 2017).
Basal CYP enzyme activities in cultured iHCs
Specific activities for human microsomal CYP1A2, 2A6, 2B6, 2C8, 2C9, 2D6, 2C19, 2E1, and 3A4 were measured in 3-day-old cultures of iHCs (Table 1). Except for CYP2A6, iHCs from a single specific donor had measurable activities for all CYP isoforms tested.
Table 1. Basal activities for major CYP isoforms (Lu, Jingtao, et al., 2015).
| iHC | iHC | pHH | |||
| Enzyme | Substrate | Metabolite | pmol/min/Million Cells | pmol/min/mg Protein | pmol/min/mg Protein (Average (Min–Max)) |
| CYP1A2 | Phenacetin | Acetaminophen | 2.8 | 19.5 | 10.6 (0–50.0) |
| CYP2A6 | Coumarin | 7-Hydroxycoumarin | ND | ND | 47.0 (20.8–137.0) |
| CYP2B6 | Bupropion | Hydroxybupropion | 0.4 | 2.3 | 15.2 (0–100.0) |
| CYP2C8 | Paclitaxel | 6α-Hydroxypaclitaxel | 0.2 | 1.0 | 8.0 (6.3–9.8) |
| CYP2C9 | Diclofenac | 4-Hydroxydiclofenac | 3.8 | 26.1 | 111.5 (0–560.0) |
| CYP2C19 | S-Mephenytoin | 4-Hydroxymephenytoin | 1.4 | 9.9 | 17.5 (0–82.3) |
| CYP2D6 | Dextromethorphan | Dextrorphan | 1.3 | 8.7 | 20.5 (0–77.6) |
| CYP2E1 | Chlorzoxazone | 6-Hydroxychlorzoxazone | 26.1 | 180.2 | 38.3 (5.0–89.0) |
| CYP3A4 | Midazolam | 1-Hydroxymidazolam | 9.1 | 62.7 | 41.9 (0–253.0) |
Induction of nuclear receptor-mediated gene transcription
Cultures were treated for 24 or 48h with omeprazole (100µM), CITCO (1µM), rifampicin (10µM), or GW7647 (1µM) and induction of CYP 1A2, CYP2B6, CYP3A4, and ACOX1 was measured by qRT-PCR. All target gene levels were expressed as the ratio to GAPDH.
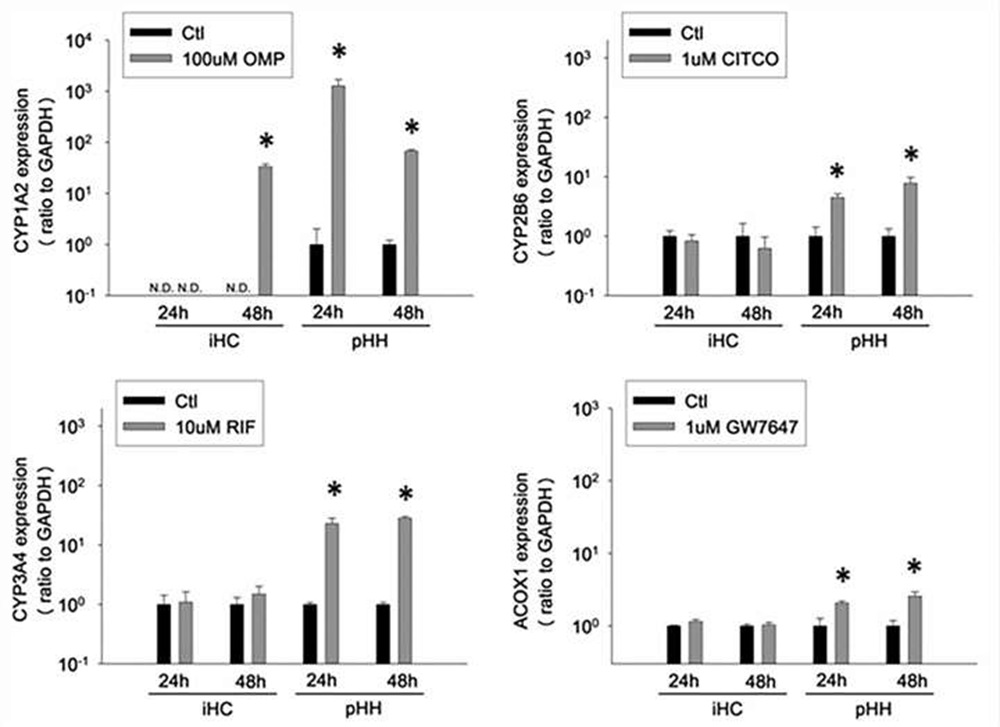 Fig. 3. Functionality of xenobiotic nuclear receptors and induction of downstream gene expression in cultured iHCs and pHHs (Lu, Jingtao, et al., 2015).
Fig. 3. Functionality of xenobiotic nuclear receptors and induction of downstream gene expression in cultured iHCs and pHHs (Lu, Jingtao, et al., 2015).
iHCs respond to prototype hepatoxic agents
The cytotoxicity of prototype hepatotoxic agents was compared in cultures of iHCs or pHHs. After a 24h exposure, iHCs and pHHs showed similar sensitivity of cell viability (ATP content) toward acetaminophen, troglitazone, and nefazodone exposure, and similar resistance to theophylline exposure.
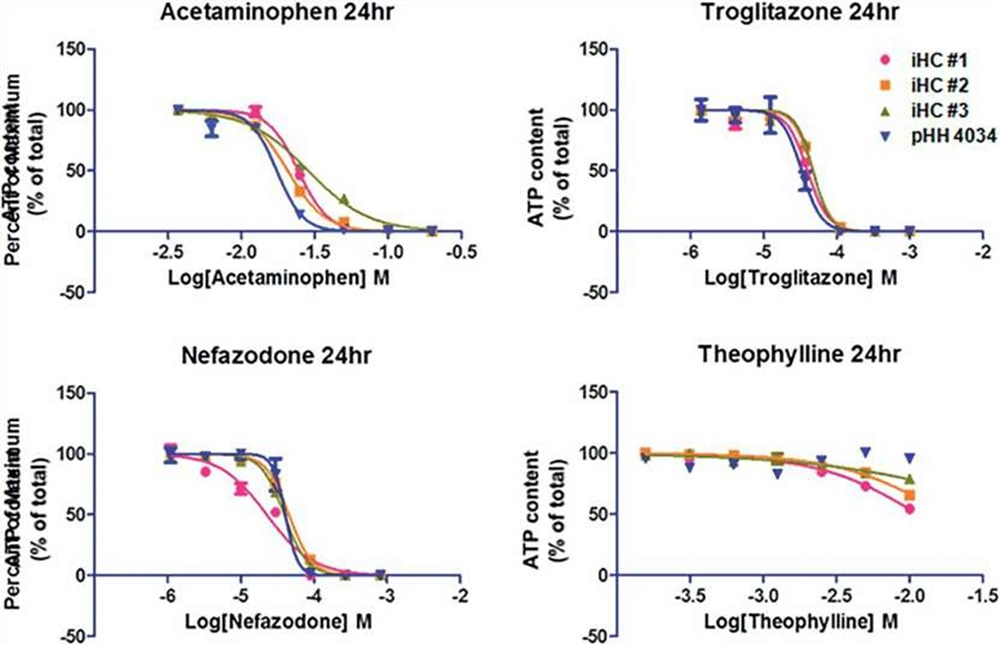 Fig. 4. Cytotoxicity of acetaminophen, troglitazone, nefazodone, theophylline in cultures of iHCs and pHHs (Lu, Jingtao, et al., 2015).
Fig. 4. Cytotoxicity of acetaminophen, troglitazone, nefazodone, theophylline in cultures of iHCs and pHHs (Lu, Jingtao, et al., 2015).
FAQ
1. What is differentiation of iPSC into hepatocytes?
The process of generating iPSC-derived hepatocytes begins with the directed differentiation of iPSC into definitive endoderm (DE) cells, which are then differentiated further into hepatocytes.
2. Why is hepatocyte differentiation from iPSC important?
It is important as it provides a valuable tool for studying liver biology, modeling liver diseases, and developing new treatments for liver-related disorders.
3. What are the methods used to differentiate iPSCs into hepatocytes?
There are several methods used to differentiate iPSCs into hepatocytes, including the use of growth factors, small molecules, and other signaling molecules to mimic the developmental process of liver cell formation.
4. How long does it take to differentiate iPSCs into hepatocytes?
The time can vary depending on the specific differentiation protocol used, but it typically takes several weeks to fully differentiate iPSCs into functional hepatocytes.
5. How can hepatocytes differentiated from iPSCs be used in research and drug development?
iPSC-derived hepatocytes can be used to study liver development, function, and disease, as well as to screen potential drug candidates for liver toxicity and efficacy.
6. Are there any challenges or limitations to hepatocyte differentiation from iPSC?
The challenges to hepatocyte differentiation from iPSC include variability in differentiation efficiency, the potential for genetic instability in iPSCs, and the need for optimization of differentiation protocols for specific research applications.
7. Can hepatocytes differentiated from iPSCs be used for cell transplantation therapy?
iPSC-derived hepatocytes have the potential to be used for cell transplantation therapy in the treatment of liver diseases, but further research is needed to optimize differentiation protocols and ensure the safety.
References
- Hay, David C., et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires Activin A and Wnt3a signaling. Proceedings of the National Academy of Sciences, 105.34 (2008): 12301-12306.
- Hay, David C., et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem cells, 26.4 (2008): 894-902.
- Agarwal, Sadhana, Katherine L. Holton, and Robert Lanza. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem cells, 26.5 (2008): 1117-1127.
- Cai, Jun, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology, 45.5 (2007): 1229-1239.
- Siller, Richard, et al. Small-molecule-driven hepatocyte differentiation of human pluripotent stem cells. Stem cell reports. 4.5 (2015): 939-952.
- Du, Cong, et al. Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails. Stem cell research & therapy, 9 (2018): 1-15.
- Mathapati, Santosh, et al. Small-molecule-directed hepatocyte-like cell differentiation of human pluripotent stem cells. Current protocols in stem cell biology, 38.1 (2016): 1G-6.
- Graffmann, Nina, Bo Scherer, and James Adjaye. In vitro differentiation of pluripotent stem cells into hepatocyte like cells–Basic principles and current progress. Stem Cell Research, 61 (2022): 102763.
- Lu, Jingtao, et al. Morphological and functional characterization and assessment of iPSC-derived hepatocytes for in vitro toxicity testing. Toxicological Sciences, 147.1 (2015): 39-54.
- Bhogal, Ricky H., et al. Isolation of primary human hepatocytes from normal and diseased liver tissue: a one hundred liver experience. PloS one, 6.3 (2011): e18222.
- Wilkening, Stefan, Frank Stahl, and Augustinus Bader. Comparison of primary human hepatocytes and hepatoma cell line Hepg2 with regard to their biotransformation properties. Drug metabolism and disposition, 31.8 (2003): 1035-1042.
- Hayhurst, Graham P., et al. Hepatocyte nuclear factor 4α (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Molecular and cellular biology, 21.4 (2001): 1393-1403.
- Schwartz, R. E., et al. Pluripotent stem cell-derived hepatocyte-like cells. Biotechnology advances, 32.2 (2014): 504-513.
Explore Other Options

