- You are here: Home
- Applications
- Cell Therapy
- CAR-T Preclinical Characterization in vivo
Applications
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
CAR-T Preclinical Characterization in vivo
Chimeric antigen receptors (CARs) are receptor proteins that are engineered to give T cells new abilities to recognize specific targets. The receptors are chimeric because they combine both antigen-binding and T-cell activating functions into a single receptor. The premise of CAR-T immunotherapy is to modify T cells to recognize cancer cells in order to more effectively target and destroy them.
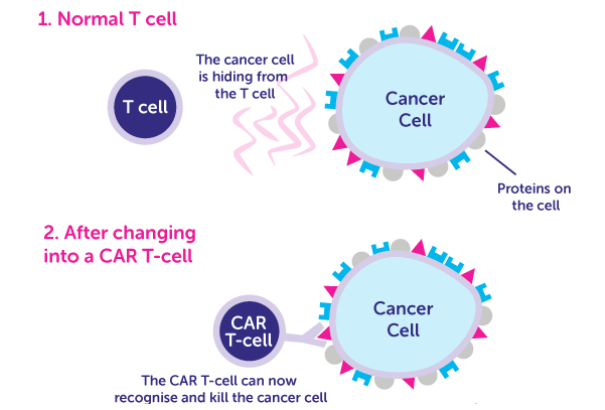
In order to apply CAR-T therapy in more conditions effectively and efficiently, preclinical assays on animal models are necessary for approval of clinical trials based on CAR-T therapy. The in vivo assays of CAR-T therapy mainly focus on characteristics, working mechanism, applicable conditions, suitable population, pathways, and target sites of CAR-T medical products.
Pharmacological, as well as Pharmacokinetic, Evaluation are Essential for CAR-T Therapies (including but not limited to)
- Target expression detection includes target binding sites and target expressing sites
- Functional analysis includes proliferation assay, cytokine secretion detection, targeting antigen distribution assay, and affinity analysis
- Anti-tumor efficacy includes tumor-killing ability detection
- In vivo Biodistribution analysis in vivo includes CAR-T exposure level detection by time, retaining duration assay, and elimination assay by time
Additionally, Safety Assessment Needs to be Carefully Monitored (including but not limited to)
- CAR-T immune response analysis involves primary dose of CAR-T cells and cytotoxicity reaction
- CAR-T cytotoxicity effect detection involves neurotoxicity, immunotoxicity, and etc.
- Risk analysis of insertional mutation involves immortalized proliferation or tumorigenicity risk
- Long-term cytotoxicity and tumorigenicity assay involve insertion site analysis
- Cytokine release syndrome (CRS) analysis involves neurotoxicity, encephaledema, and etc.
- Safety analysis of virus vector involves type, biodistribution, proliferating level etc. of virus
- Risk of choice for model animals involves normal or disease-model animals
All of the in vivo preclinical characterization share the same principle: integration of pharmacological, pharmacokinetic, and safety analysis to establish the most efficient and cost-saving experiment for CAR-T medical products and CAR-T cells.
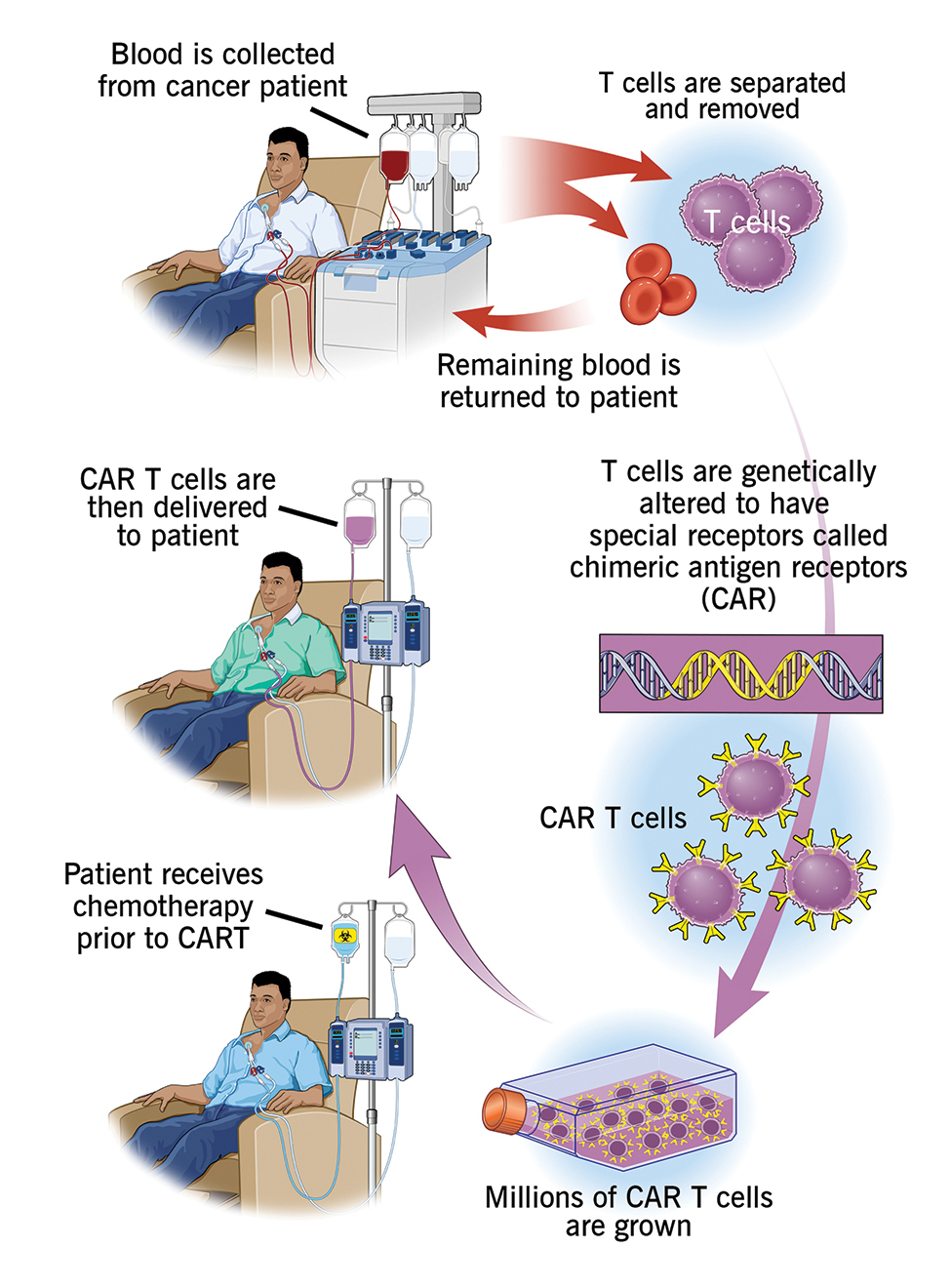
Creative Bioarray is an experienced and outstanding provider of CAR-T Preclinical Characterization. We are dedicated to providing detailed data and comprehensive service for your scientific research, and we are pleased to use our advanced platform and professional experience to satisfy your demand for you.

If you have any special need in CAR-T Preclinical Characterization, do not hesitate to contact us for this special service. Please let us know what you need and we will accommodate you. We are looking forward to working with you in the future.
Explore Other Options
For research use only. Not for any other purpose.

