- You are here: Home
- Disease Models
- Inflammation & Autoimmune Disease Models
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Pain Models
- Metabolic Disease Models
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
Experimental Autoimmune Myasthenia Gravis (EAMG) Model
Creative Bioarray provides in vivo pharmacology studies using a well-validated Experimental Autoimmune Myasthenia Gravis (EAMG) animal model. This model is designed to quickly assess the effectiveness of test compounds. Our scientific team is committed to working closely with you, from the initial study design through the final data analysis and reporting. By partnering with us, you can anticipate consistent and dependable outcomes for your research needs.
Myasthenia gravis (MG) is an autoimmune disorder characterized by the body's immune system attacking the acetylcholine receptor (AChR), muscle-specific kinase (MuSK), or other AChR-related proteins in the postsynaptic muscle membrane. This condition predominantly affects the voluntary muscles, particularly those governing the eyes, mouth, throat, and limbs. MG can occur at any age but is more prevalent among young women (ages 20 to 30) and men over 50. The EAMG model has significantly contributed to our understanding of the disease's pathophysiology, highlighting the roles of specific autoantibodies and T helper lymphocytes. The model, which can be induced in susceptible mice and rats to exhibit symptoms akin to those seen in humans, is invaluable for studying both muscular and immune system aspects of MG. It offers a platform for evaluating new treatment strategies, and suggesting potential avenues for disease prevention and modulation.
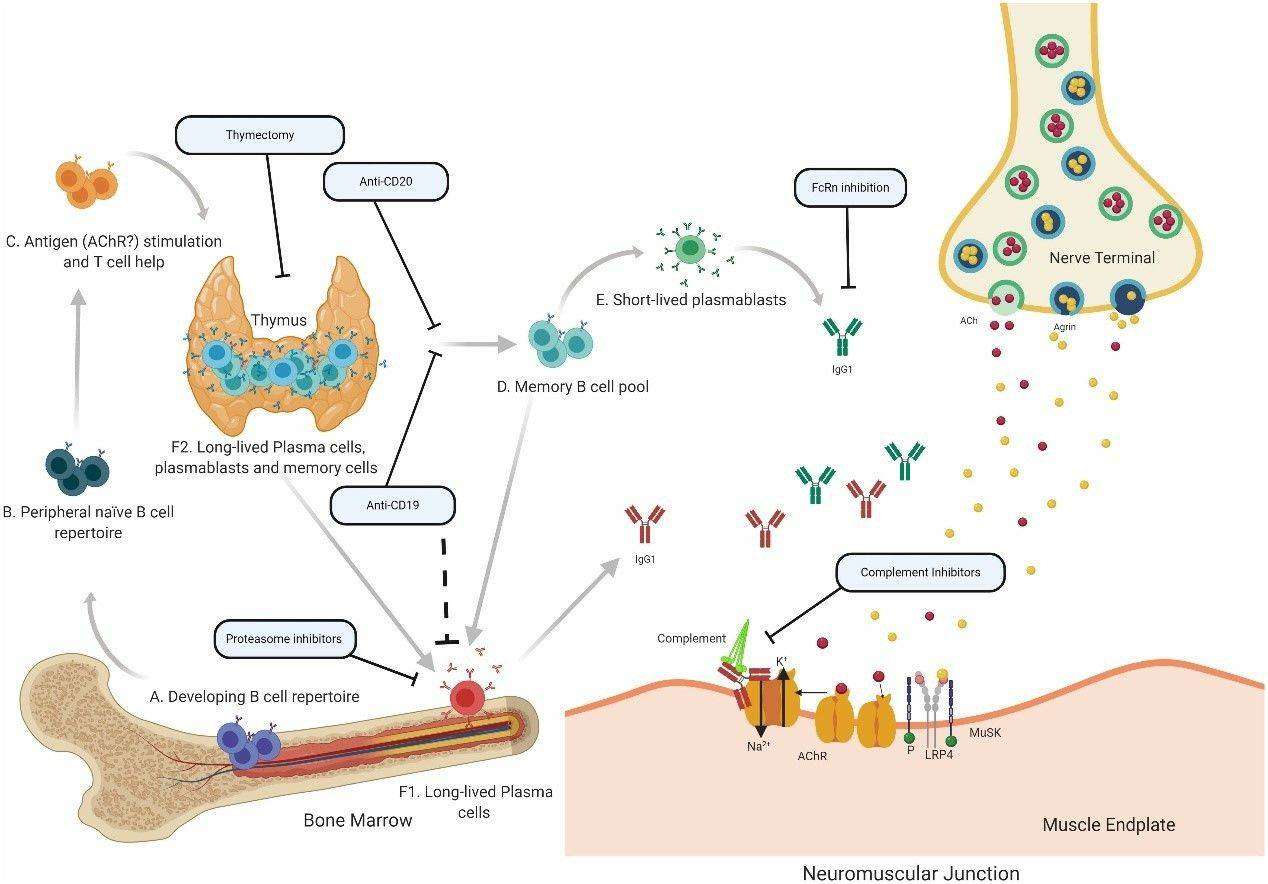 Fig. 1 Speculative mechanisms of AChR MG immunopathology. (Fichtner et al. 2020)
Fig. 1 Speculative mechanisms of AChR MG immunopathology. (Fichtner et al. 2020)
Our Experimental Autoimmune Myasthenia Gravis (EAMG) Model
- Available Animal
- Rat
- Mouse
- Modeling Method
Creative Bioarray has successfully developed an EAMG model in animals. This model is induced through immunization with a synthetic peptide that corresponds to amino acids 97-116 of the α subunit of the rat acetylcholine receptor (AChR), known as the R97-116 peptide.
- Endpoints
- Body weight
- Histology analysis: H&E staining
- Cytokine analysis
- Anti-AChR-antibody analysis
- qPCR or Western blot
- Other customized endpoints
Example Data
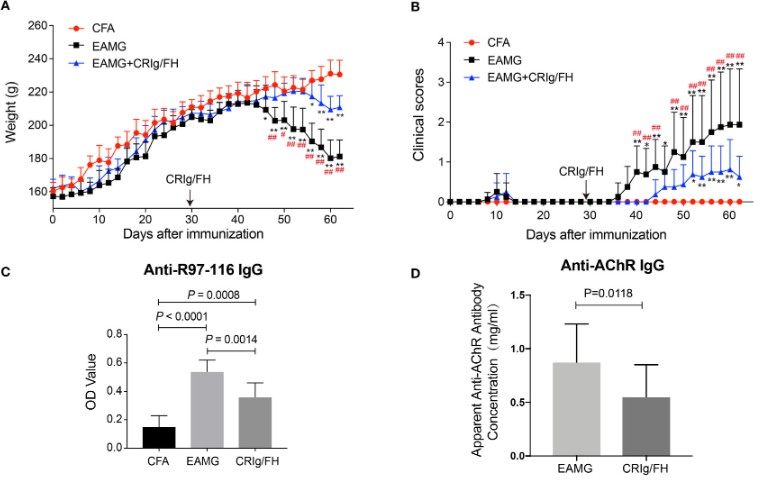 Fig. 2 CRIg/FH treatment ameliorates active EAMG. (A, B) CRIg/FH ameliorates active EAMG clinical outcomes significantly. The body weights (A) and clinical scores (B) were recorded every other day. (C, D) CRIg/FH reduces the serum levels of auto-antibodies. The serum antibody against AChR 97-116 peptide was measured by ELISA (C) and the apparent anti-AChR antibody concentration was determined by immunofluorescence with H9c2(2-1) cells expressing rat muscle AChR, corresponding to a concentration of mAb35, which gives an equivalent fluorescent signal on H9C2(2-1) cells (D). (Song et al. 2022)
Fig. 2 CRIg/FH treatment ameliorates active EAMG. (A, B) CRIg/FH ameliorates active EAMG clinical outcomes significantly. The body weights (A) and clinical scores (B) were recorded every other day. (C, D) CRIg/FH reduces the serum levels of auto-antibodies. The serum antibody against AChR 97-116 peptide was measured by ELISA (C) and the apparent anti-AChR antibody concentration was determined by immunofluorescence with H9c2(2-1) cells expressing rat muscle AChR, corresponding to a concentration of mAb35, which gives an equivalent fluorescent signal on H9C2(2-1) cells (D). (Song et al. 2022)
Quotation and Ordering
Creative Bioarray boasts state-of-the-art pre-clinical technology platforms and a team of seasoned researchers and scientists. This combination enables us to offer an extensive array of services while maintaining consistently high-quality results and ensuring quick turnaround times. Our advanced capabilities and expert personnel make us a reliable partner for various pre-clinical and research needs, delivering precise and efficient outcomes across diverse projects.
References
- Fichtner, M.L., et al. Autoimmune pathology in myasthenia gravis disease subtypes is governed by divergent mechanisms of immunopathology. Frontiers in immunology, 2020, 11: 776.
- Mantegazza, R., et al. Animal models of myasthenia gravis: utility and limitations. Int J Gen Med, 2016;9:53-64.
- Song, J., et al. A targeted complement inhibitor CRIg/FH protects against experimental autoimmune myasthenia gravis in rats via immune modulation. Frontiers in Immunology, 2022, 13: 746068.
For research use only. Not for any other purpose.

