- You are here: Home
- Disease Models
- Oncology Models
- Cancer Vaccine Evaluation
- Cancer Vaccine Evaluation in Humanized MHC&TAP Mouse
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Pain Models
- Metabolic Disease Models
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
Cancer Vaccine Evaluation in Humanized MHC&TAP Mouse
Creative Bioarray has successfully developed the Humanized MHC&TAP Mouse Model, a cutting-edge platform designed to enhance the accuracy of preclinical cancer vaccine research. By meticulously replacing the endogenous mouse MHC and TAP molecules with their human counterparts, our team has created a model that closely mimics the antigen presentation process of the human immune system. This innovative approach not only ensures a more accurate reflection of human immune responses but also significantly enhances the reliability of vaccine efficacy and safety evaluations. With this model, Creative Bioarray offers our clients a powerful tool to bridge the gap between preclinical and clinical vaccine development, thereby accelerating the pace of vaccine innovation and improving the success rate of vaccine candidates transitioning into human trials.
Applications
Advantages
- Improved Antigen Presentation
This model demonstrates superior capabilities in presenting antigens, fostering optimal immune system responses.
- Dual Humanization of MHC and TAP
The strength of this approach is in the humanization of both MHC and TAP molecules. The humanized TAP molecules enhance antigen peptide recognition by selectively interacting with sequences rich in hydrophobic, basic, and aromatic amino acids.
- Varied Antigen Delivery
Discover the ability to present a wide range of antigens, such as peptides and proteins (including RNA and DNA), while effectively screening naturally occurring epitopes.
- Strong Epitope Selection
Experience exceptional proficiency in epitope screening that is supported by high efficiency and accuracy.
Example Data
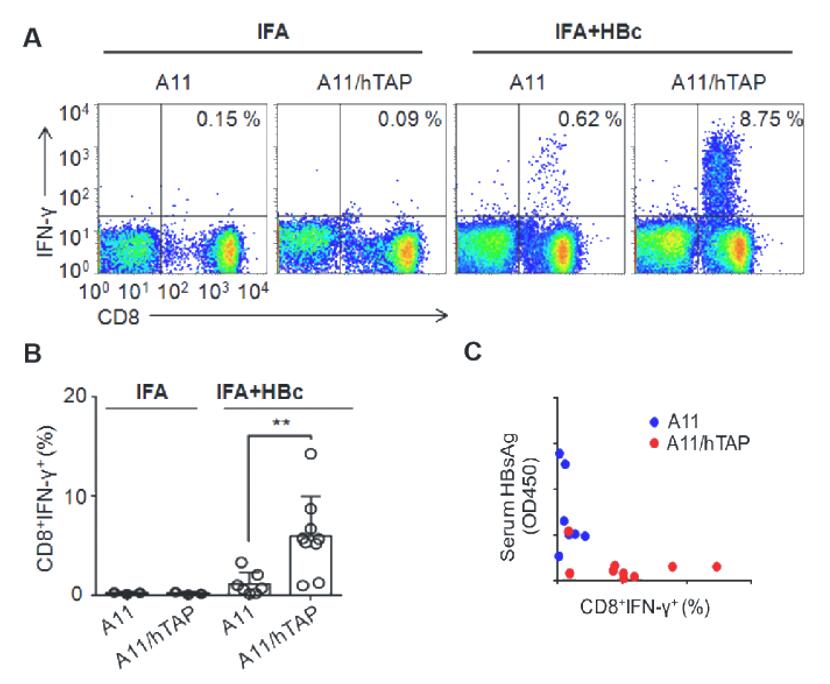 Fig. 1 HBV long peptide vaccine induces a protective CTL immune response in the new generation of transgenic mice. Using the new generation HLA-A11 mice, an HBV infection model was established, and long peptide immunization was performed to further validate the application of these mice in T-cell immunity. The results show that the new generation HLA-A11 mice generated a strong HBV-specific CTL immune response and effectively reduced the HBsAg levels in the body.
Fig. 1 HBV long peptide vaccine induces a protective CTL immune response in the new generation of transgenic mice. Using the new generation HLA-A11 mice, an HBV infection model was established, and long peptide immunization was performed to further validate the application of these mice in T-cell immunity. The results show that the new generation HLA-A11 mice generated a strong HBV-specific CTL immune response and effectively reduced the HBsAg levels in the body.
Quotation and Ordering
Creative Bioarray's state-of-the-art pre-clinical evaluation platform for cancer vaccines provides an extensive array of robust tools and comprehensive support, tailored to meet the complex needs of your research endeavors. Our platform is meticulously designed to facilitate a thorough assessment of vaccine efficacy, safety, and immunogenicity, ensuring that your cancer vaccine candidates are rigorously tested in a controlled environment that closely simulates clinical conditions. If you are interested in our services, please feel free to contact us at any time or submit an inquiry to us directly.
For research use only. Not for any other purpose.

