- You are here: Home
- Disease Models
- Inflammation & Autoimmune Disease Models
- Sjögren's Syndrome Model
- Experimental Sjögren's Syndrome Model
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Pain Models
- Metabolic Disease Models
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
Experimental Sjögren's Syndrome Model
Creative Bioarray possesses extensive experience in Sjögren's Syndrome research. Our dedicated team will collaborate closely with you, guiding you through every step of the process, from the selection of appropriate models and the design of study plans to the final data analysis and reporting. We have successfully established a stable Experimental Sjögren's Syndrome Model, which is instrumental in evaluating the efficacy of your drug candidates. Our commitment is to provide you with comprehensive support and reliable services to facilitate your research and development endeavors in the field of Sjögren's Syndrome.
Sjögren's Syndrome (SS) is a systemic autoimmune disease characterized primarily by the infiltration of lymphocytes into exocrine glands. This condition predominantly manifests as symptoms of dry mouth and dry eyes. The principal mechanism behind the development of SS is believed to involve the destruction of the exocrine gland epithelium, mediated by an abnormal response of immunocytes to autoantigens. Currently, there are no ideal drugs specifically designed for the treatment of SS. Treatment strategies primarily focus on symptomatic relief and systemic therapy, aimed at alleviating symptoms and slowing the progression of the disease. However, systemic therapies, including the use of glucocorticoids or immunosuppressive treatments, can lead to adverse effects such as gastrointestinal ulcers, immunosuppression, and even the development of tumors. Consequently, the quest for safe and effective therapeutic options for SS remains a critical research focus.
Our Experiment Sjögren's Syndrome Model
Animal Species
- Mouse
Modeling Method
- Submandibular gland (SG) proteins are prepared by homogenizing the bilateral submandibular glands of C57BL/6 mice. The SG protein is then used for immunization on days 0, 7, and 14 to induce an experimental Sjögren's syndrome model.
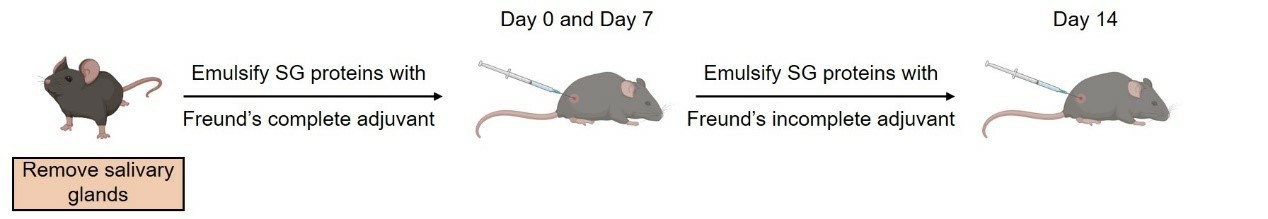 Fig. 1 Modeling method of Experiment Sjögren's Syndrome Model
Fig. 1 Modeling method of Experiment Sjögren's Syndrome Model
Endpoints
- Body weight
- Salivary flow rate
- Salivary gland index
- Histology analysis: H&E staining
- Cytokine analysis
- Other customized endpoints: according to your specific needs
Example Data
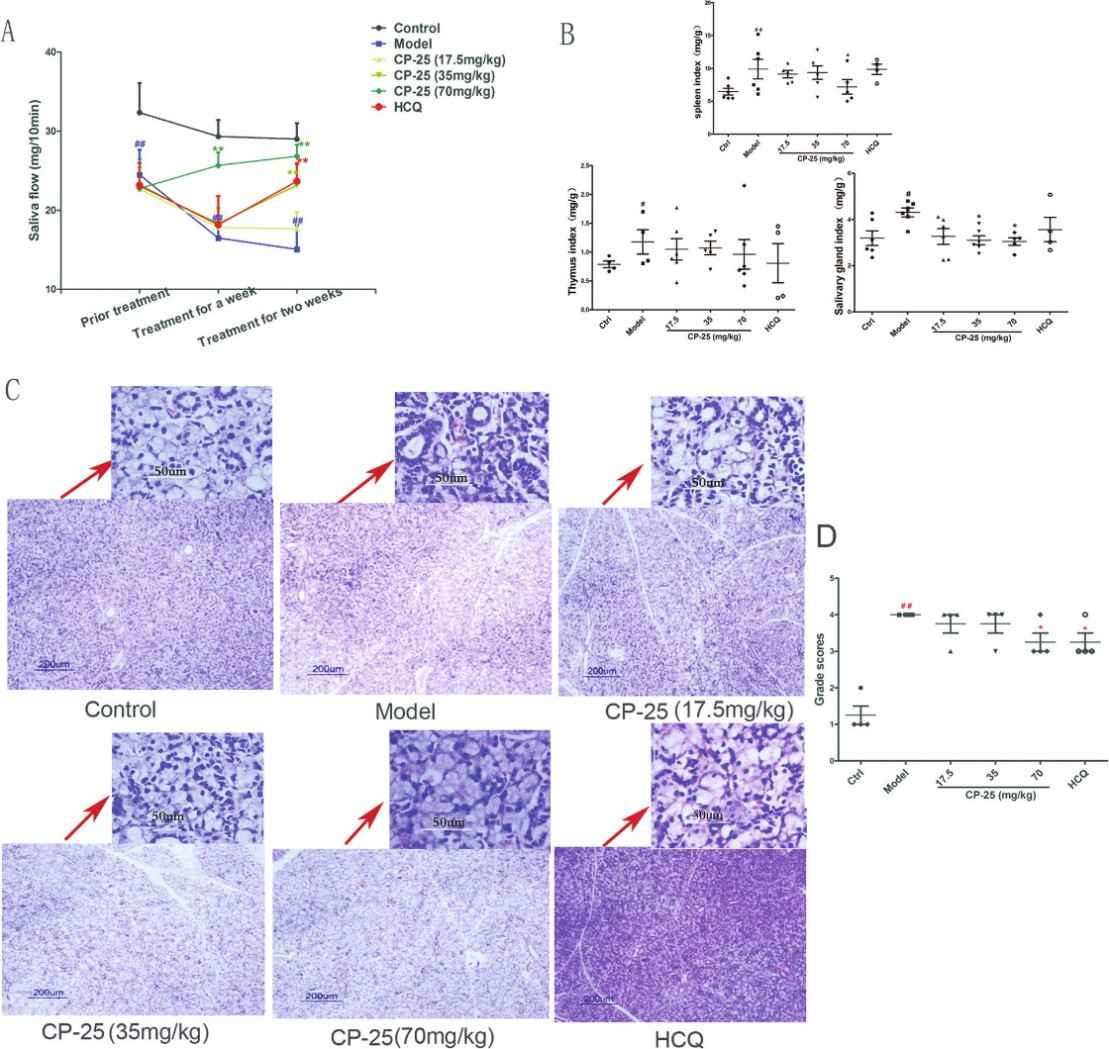 Fig. 2 The effects of CP-25 on saliva flow, the salivary gland indexes, and histological assessment in SS mice. (A) CP-25 (35, 70 mg/kg) increased saliva flow in SS mice after a 2-week treatment. (B) CP-25 reduced the salivary gland indexes in SS mice. (C, D) CP-25 attenuated lymphocytic infiltration in the salivary gland and significantly improved histological scores in SS mice. (Wu et al.2021)
Fig. 2 The effects of CP-25 on saliva flow, the salivary gland indexes, and histological assessment in SS mice. (A) CP-25 (35, 70 mg/kg) increased saliva flow in SS mice after a 2-week treatment. (B) CP-25 reduced the salivary gland indexes in SS mice. (C, D) CP-25 attenuated lymphocytic infiltration in the salivary gland and significantly improved histological scores in SS mice. (Wu et al.2021)
Quotation and Ordering
Creative Bioarray boasts a team of scientists with profound therapeutic expertise and extensive experience in the development and validation of models. We are committed to offering our clients the most dependable services at the most competitive prices. If you are interested in our services, please feel free to contact us at any time or submit an inquiry to us directly.
Reference
- Wu, H., et al. CP-25 alleviates antigen-induced experimental Sjögren's syndrome in mice by inhibiting JAK1-STAT1/2-CXCL13 signaling and interfering with B-cell migration. Laboratory Investigation, 2021, 101(8): 1084-1097.
For research use only. Not for any other purpose.

