- You are here: Home
- Disease Models
- Oncology Models
- Cancer Vaccine Evaluation
- Cancer Vaccine Evaluation in Immunocompetent Mouse
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Pain Models
- Metabolic Disease Models
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
Cancer Vaccine Evaluation in Immunocompetent Mouse
Immunocompetent mice, equipped with a complete immune system, are essential for evaluating vaccine effectiveness and immune reactions in pharmaceutical research. At Creative Bioarray, our skill in handling and caring for animals enables us to apply diverse immunization methods suited to particular vaccines or antigens. By employing advanced techniques like ELISpot assays and flow cytometry, we carefully assess the generation of cytotoxic T lymphocytes (CTLs), vital for the body's cellular immune defense. This meticulous process allows us to accurately distinguish the immune-stimulating capabilities of different vaccines. With our specialized expertise and experience in immunological assessments using immunocompetent mouse models, Creative Bioarray provides comprehensive and precise evaluations, guaranteeing the dependability and accuracy of vaccine development procedures.
Workflow
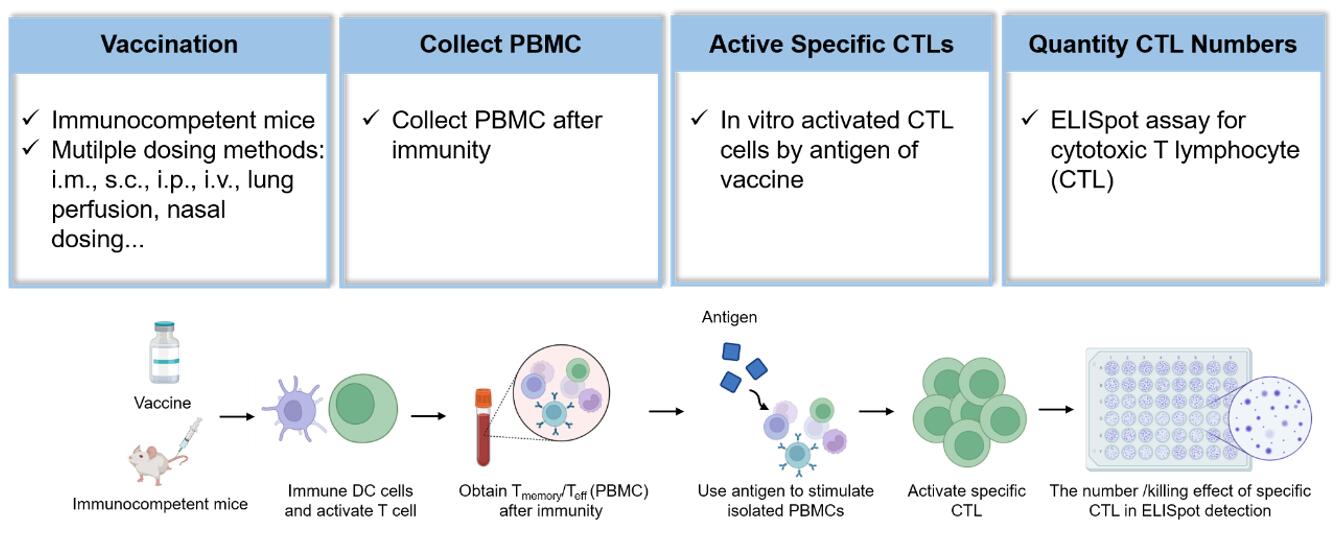 Fig. 1 Workflow for evaluating cancer vaccines in immunocompetent mouse.
Fig. 1 Workflow for evaluating cancer vaccines in immunocompetent mouse.
Applications
- Vaccine sequence screening
- Antigenic peptide screening
- Adjuvant screening
- Testing and optimizing vaccine administration schedules
- In vivo efficacy studies
Example Data
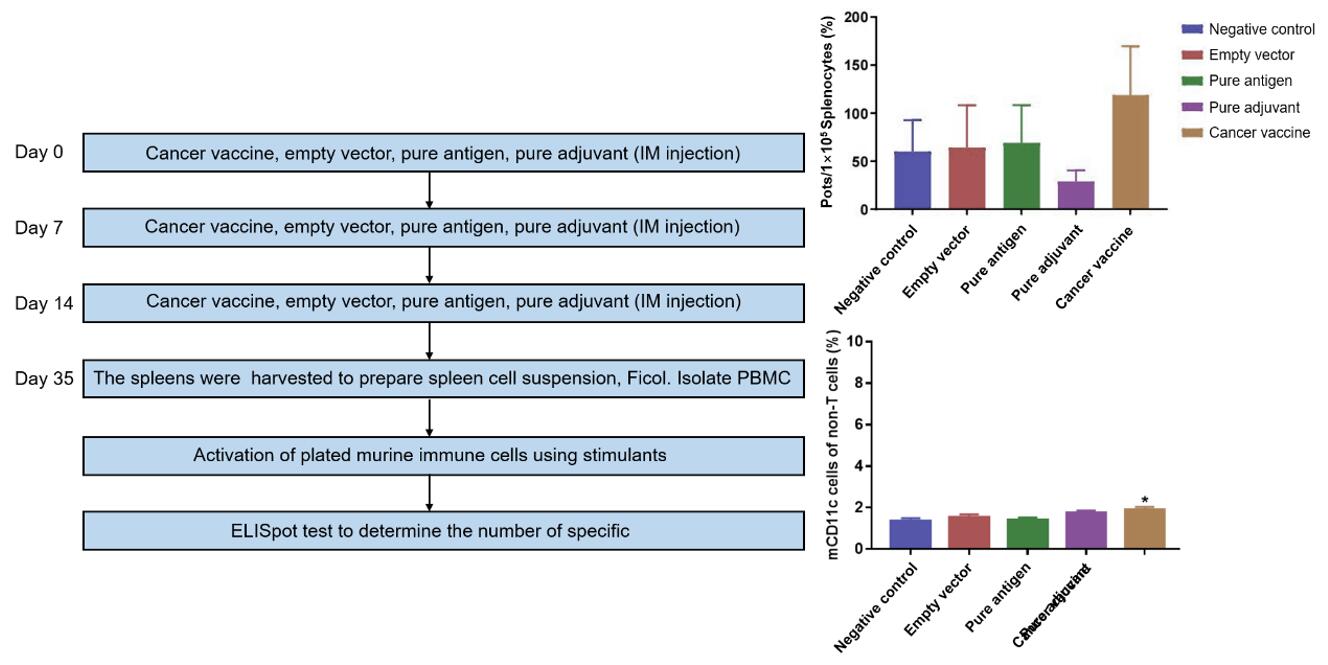 Fig. 2 Intramuscular cancer vaccine immunization. After intramuscular injection of cancer vaccine, mouse immune cells can be activated by stimulators. Mouse immune cells after immunization with empty vector, pure antigen and adjuvant cannot be activated by activators to produce CTL. At the same time, in the cancer vaccine immunization group, DC cells also increased significantly after the mouse immune cells were activated by stimulants.
Fig. 2 Intramuscular cancer vaccine immunization. After intramuscular injection of cancer vaccine, mouse immune cells can be activated by stimulators. Mouse immune cells after immunization with empty vector, pure antigen and adjuvant cannot be activated by activators to produce CTL. At the same time, in the cancer vaccine immunization group, DC cells also increased significantly after the mouse immune cells were activated by stimulants.
Quotation and Ordering
Creative Bioarray specializes in conducting preclinical studies for cancer vaccines, employing sophisticated and refined models to ensure the highest level of accuracy and reliability. Our expertise lies in evaluating the safety, efficacy, and immunological responses of these vaccines, utilizing advanced techniques and methodologies to simulate human immune responses as closely as possible. If you are interested in our services, please feel free to contact us at any time or submit an inquiry to us directly.
For research use only. Not for any other purpose.

