- You are here: Home
- Applications
- Oncology
- Translational Oncology
- Drug Efficacy Evaluation
Applications
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
Drug Efficacy Evaluation

The progress from drug discovery to its commercialization is complex, lengthy and expensive. Before a new drug comes to market, it should be tested and evaluated from several ways as follows:
Drug efficacy evaluation provides an analytic framework for making better decisions about a new drug’s appropriate place in therapy.
Many experimental cancer models have been developed to investigate carcinogenesis, cancer progression, metastasis, and other aspects in oncology and these models turned out to be useful in the efficacy evaluation and the safety prediction of oncology drugs.
Creative Bioarray offers several assays to evaluate a compound's effects on tumor cells in vitro, including cell cycle assays, proliferation, apoptosis, migration & invasion, cytotoxicity, and cell-based drug discovery services. We also perform a number of evaluation assays in vivo. Using various tumor cell lines, primary cultured cells, and animal tumor models, we examine causes of cell viability and metabolism by compounds or drugs to investigate mechanisms of efficiency and toxicity of pharmaceuticals. We conduct experiments using multiple advanced analysis systems and technologies, such as flow cytometers, laser scanning cytometers, laser capture microdissection systems, real-time PCR systems, and DNA microarray analysis systems.
Within decades of research experience on drug efficacy evaluation, Creative Bioarray is always ready to provide study design and experiment for customers to facilitate their research on drug discovery and screening.
Advantages
- Get robust results with a fast turnaround time
- Obtain rich data generated by our dedicated team of scientists
- Satisfy different demands using various cell lines and diversified advanced analysis systems
Workflow

Study examples
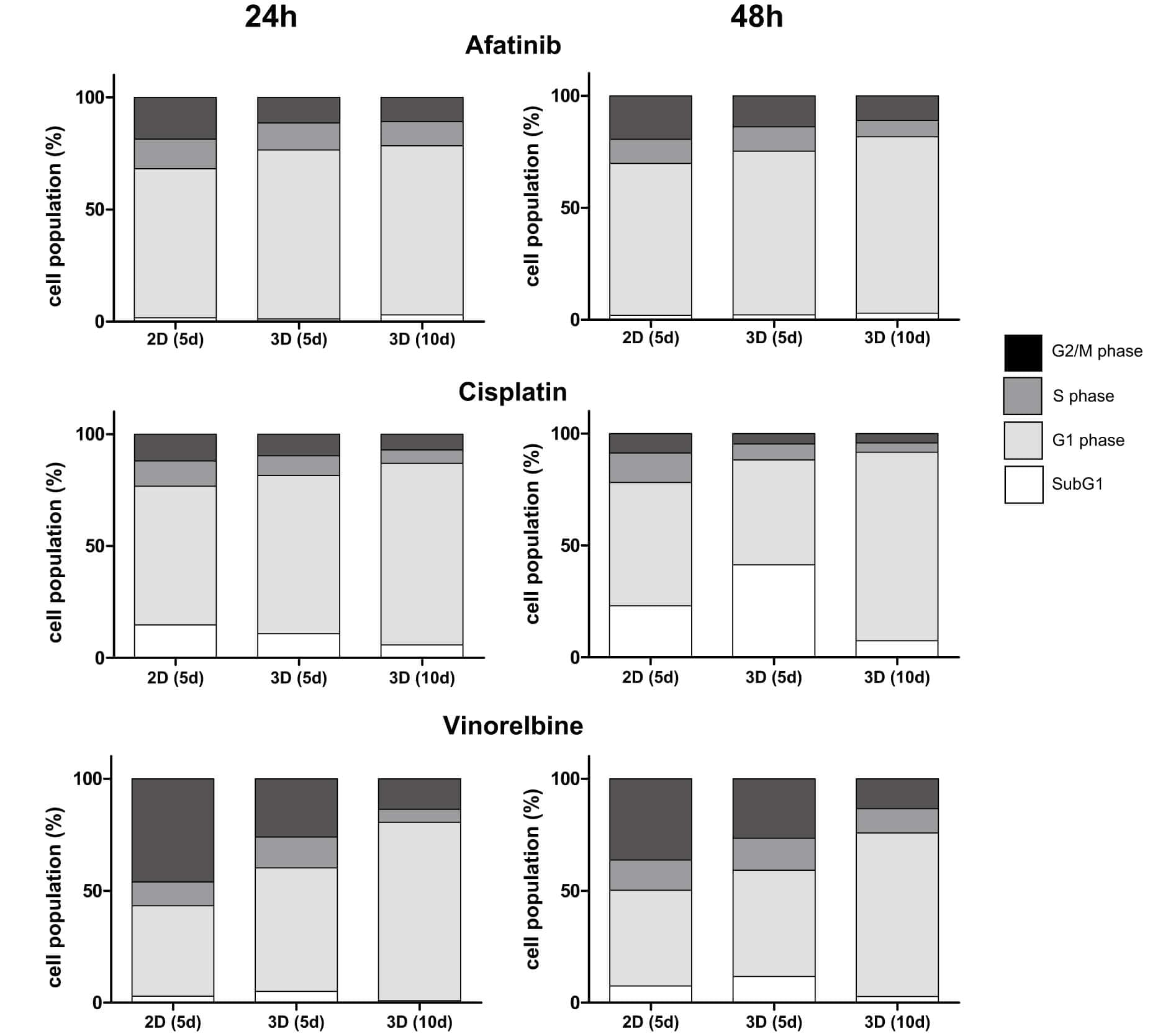
Fig.1 Determination of cytostatic effect: cells and microtissues are treated with increasing doses of afatinib, cisplatin and vinorelbine for 24 and 48 h.
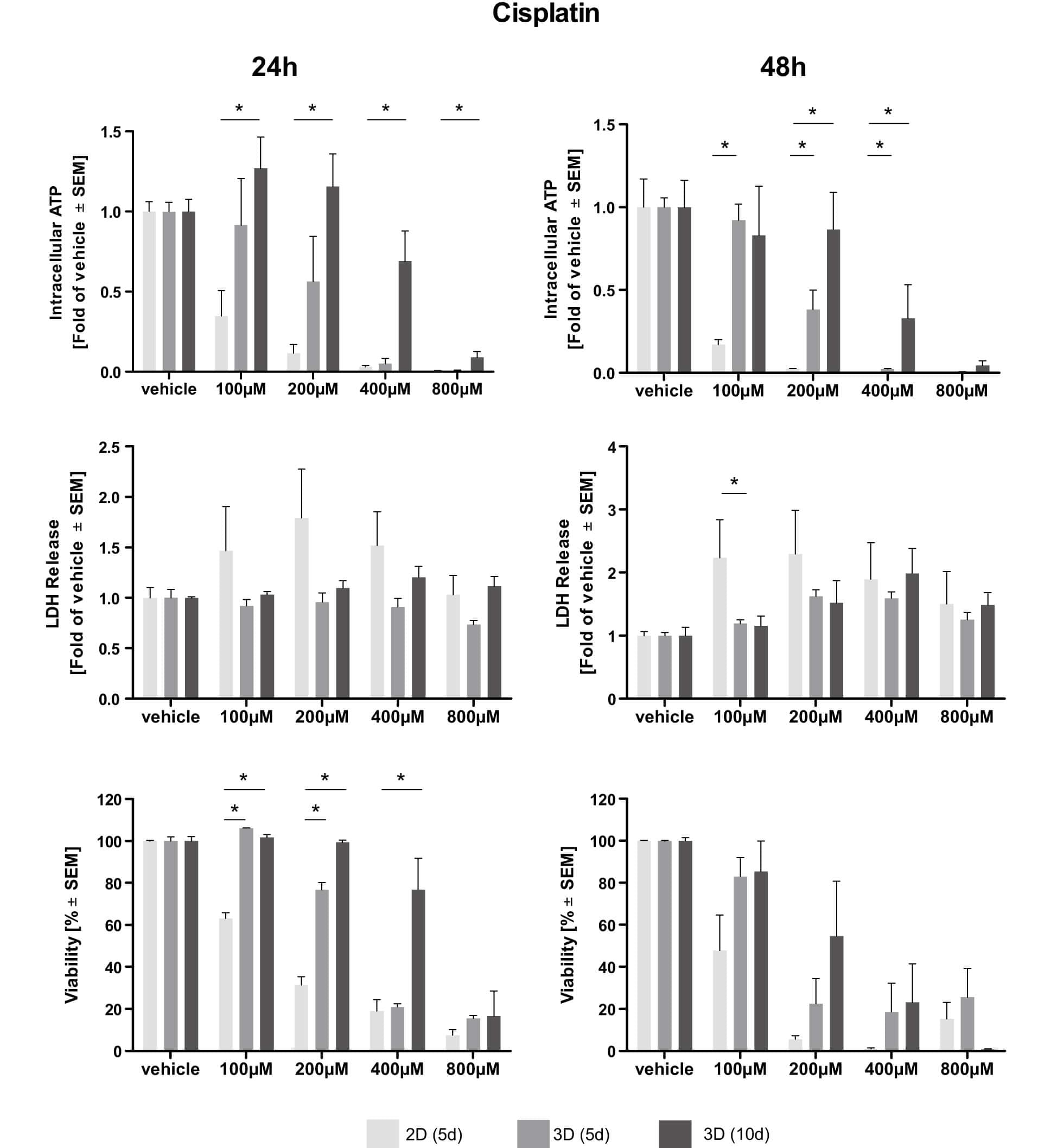
Fig.2 Dose–response curves of cisplatin
Quotation and ordering
If you have any special needs in Drug Efficacy Evaluation, please contact us for this special service. Let us know what you need and we will accommodate you. We look forward to working with you in the future.
References
| 1. | Peglere, S.; Underhill, J. Evaluating the safety and effectiveness of new drugs. American Family Physician. 2010, 82(1): 53-57. |
| 2. | Huber, J.M.; et al. Evaluation of assays for drug efficacy in a three-dimensional model of the lung. J Cancer Res Clin Oncol. 2016, 142: 1955–1966. |
| 3. | Hayakawa, Y.; et al. Report on the use of non-clinical studies in the regulatory evaluation of oncology drugs. Cancer Sci. 2016, 107: 189–202 |
Explore Other Options
For research use only. Not for any other purpose.

