- You are here: Home
- Services
- Cell Services
- 3D-Based Services
- 3D Hepatotoxicity Service
Services
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
3D Hepatotoxicity Service
Drug-induced liver injury (DILI) is the leading cause of post-market drug withdrawals and the main challenge in drug development for the accurate prediction of the potential hepatotoxic nature of new pharmaceuticals of in vivo toxicity. The traditional methods for testing DILI are based on animal models and 2D gold standard monolayer culture systems. However, it is reported that less than half of animal tests can correctly predict the toxic effects because of the species-specific differences in hepatic metabolism and different sensitivity to toxic effects between experimental species and humans. Moreover, hepatocytes de-differentiate and rapidly lose their morphology within a few days, lose their liver specific functions, such as detoxification, enzymes activity, and the production of plasma proteins like albumin under 2D culture conditions. Thus, the relevance of such tests is being questioned nowadays. 3D culture systems bridge the gap between the clinical trials and traditional 2D tests, as 3D culture systems for hepatocytes maintain the hepatocyte function and delay the de-differentiation which are especially important during drug screening processes. The interaction of hepatocytes with a 3D environment preserves their function including drug metabolism, transport, and CYP induction. Therefore detection and prediction of hepatotoxicity by using 3D culture is an important approach in order to minimize the occurrence of DILI.
Creative Bioarray 3D hepatotoxicity assay service advantages
- Providing novel approaches to in vitro drug safety assessment that overcomes many limitations of existing models.
- Several physiologic model systems are available by using primary cells, hepatic cell lines or iPSC.
- Our liver toxicity tests cover most endpoints.
- The detection includes both acute and chronic toxicity.
- Spheroids of hepatocytes co-cultured with non-parenchymal cells better mimic the complex structure of human liver.
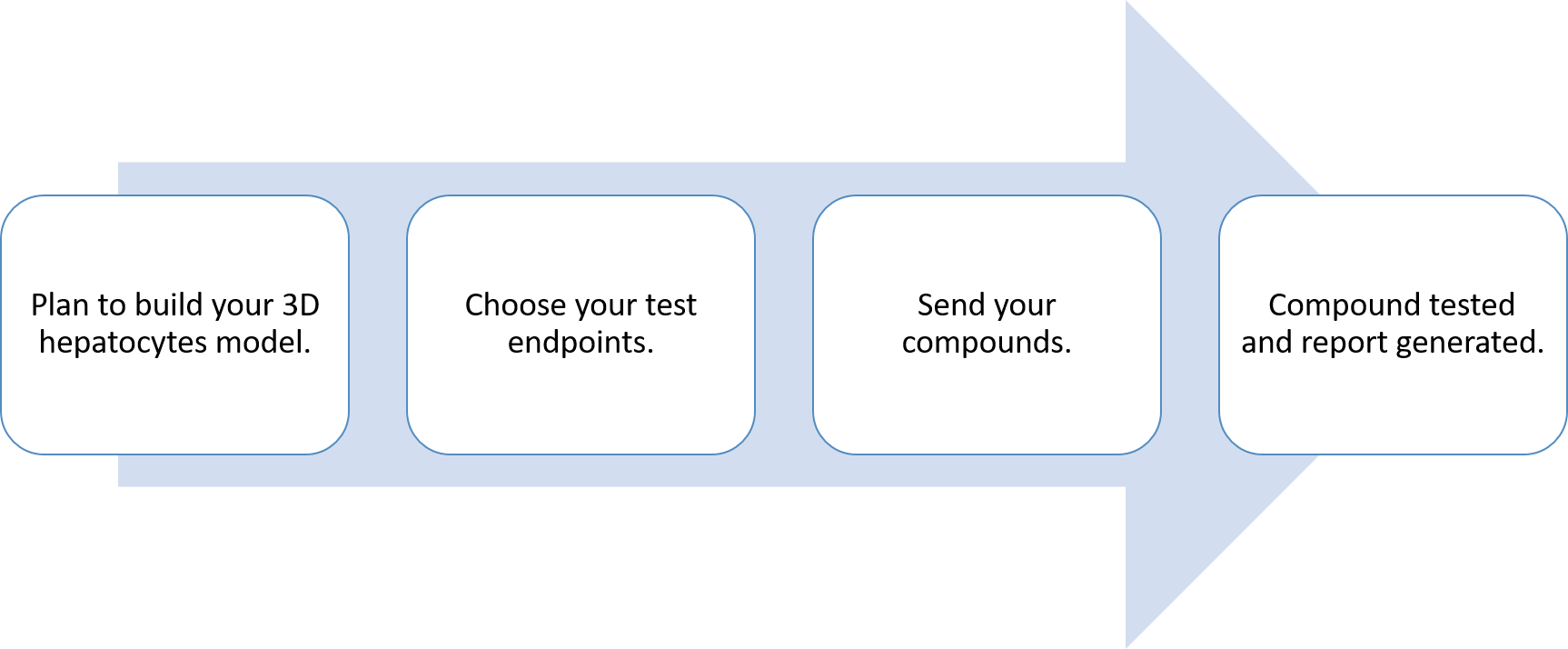
Workflow
Applications
Hepatocytes in 3D culture are the proper culture models for testing the liver-specific functions in vitro. The functional parameters such as CYP activity, ATP, albumin production, and enzyme content, etc., will be measured based on the customers’ choices. Albumin and urea are synthesized by the liver. As so, they are markers for the ability of the liver to synthesize proteins. CYPs are involved in the metabolism of drugs, chemicals and endogenous substrates. CYP-mediated activation of drugs to toxic metabolites induces hepatotoxicity. Thus, a wide range of assays for liver in vitro models will evaluate your compounds by characterization hepatic functions and their drug-mediated responses.
Study examples
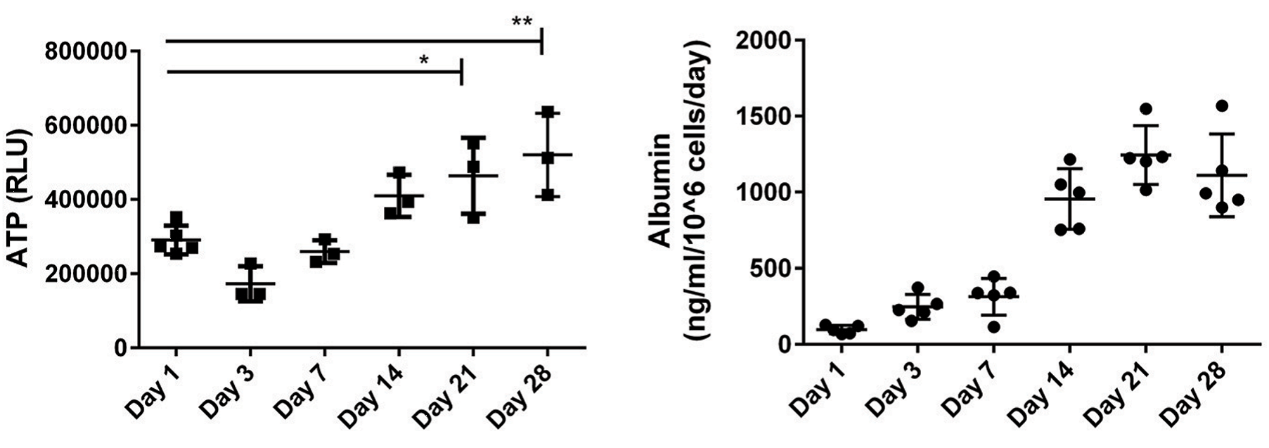 Figure. The representative data analysis for the measurements of tissue ATP and the secreted factor albumin when compared 2D culture to 3D culture.
Figure. The representative data analysis for the measurements of tissue ATP and the secreted factor albumin when compared 2D culture to 3D culture.
Quotations and ordering
Our customer service representatives are available 24hr a day!
References
- Bachmann, A., et al. 3D Cultivation Techniques for Primary Human Hepatocytes. Microarrays. 2015, 4.1: 64-83.
- Nguyen, D. G., et al. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS One. 2016, 11.7: e0158674.
- Gunness, P., et al. 3D organotypic cultures of human HepaRG cells: a tool for in vitro toxicity studies. Toxicological sciences. 2013, 133.1: 67-78.
Explore Other Options
For research use only. Not for any other purpose.

