Nasopharyngeal Tumor Cells
- Background
- Applications
- Scientific Data
- FAQ
Nestled in the upper region of the pharynx, the nasopharynx is a vital part of the human anatomy that plays a crucial role in respiratory and auditory functions. Nasopharyngeal carcinoma (NPC) is a rare and aggressive form of head and neck cancer with a distinct geographical distribution. Nasopharyngeal tumor cells originate from the epithelial lining of the nasopharynx, a region located at the back of the nasal cavity.
Cellular Characteristics of Nasopharyngeal Tumor Cells
- Histological classification. Nasopharyngeal tumor cells can be broadly classified into two main histological subtypes: undifferentiated and differentiated. The undifferentiated subtype is characterized by a complete lack of cellular differentiation, resulting in a highly aggressive and rapidly proliferating phenotype. These tumor cells exhibit a pleomorphic appearance, with large, irregular nuclei and a high nuclear-to-cytoplasmic ratio, often appearing as a homogeneous sheet of cells under the microscope. In contrast, the differentiated subtype displays a more organized cellular structure, with varying degrees of squamous or glandular differentiation. These tumor cells tend to exhibit a lower proliferative rate and a more favorable prognosis compared to their undifferentiated counterparts.
- Immunohistochemical profiling. The overexpression of cytokeratins, such as CK5/6 and CK19, is a hallmark of nasopharyngeal tumor cells, reflecting their epithelial origin. Additionally, the presence of Epstein-Barr virus (EBV)-encoded latent membrane protein 1 (LMP1) and Epstein-Barr nuclear antigen 1 (EBNA1) in the majority of these tumor cells underscores the critical role of the EBV in their pathogenesis.
- Molecular characteristics. Through cutting-edge genomic analyses, some research has identified a complex landscape of genetic aberrations, including recurrent mutations in genes such as TP53, KRAS, and PIK3CA, as well as chromosomal abnormalities like 3q and 12p amplifications. Importantly, the deregulation of key signaling pathways, such as the PI3K/AKT/mTOR, MAPK, and Wnt/β-catenin cascades, has been widely documented in nasopharyngeal tumor cells. These molecular alterations not only contribute to the aggressive nature of these tumors but also present potential targets for the development of personalized therapeutic strategies.
Development of Targeted Therapies
Nasopharyngeal tumor cells are used to identify novel therapeutic targets and develop targeted therapies. For instance, mutations in the PIK3CA gene, which encodes a protein involved in cell growth and survival, have been identified in nasopharyngeal tumor cells. Inhibitors targeting the PI3K/AKT signaling pathway are currently under investigation as potential treatments for NPC.
Biomarker Discovery
The analysis of nasopharyngeal tumor cells has led to the identification of biomarkers that can be used for diagnosis, prognosis, and treatment stratification. EBV-encoded small RNA (EBER) and viral capsid antigen (VCA) are examples of EBV-related biomarkers that are being evaluated for their utility in NPC detection and monitoring.
Tumor Microenvironment Research
Understanding the interactions between nasopharyngeal tumor cells and their microenvironment is crucial for developing new treatments. Several studies investigate the role of stromal cells, immune cells, and extracellular matrix components in tumor growth and progression.
Drug Resistance and Sensitivity Testing
Nasopharyngeal tumor cells are used to study drug resistance mechanisms and identify novel combination therapies. High-throughput screening assays are conducted to help in the identification of drugs that can overcome resistance and improve patient outcomes.
Single-Cell Transcriptomic Profiling of the Multicellular Ecosystem of NPC
To systematically survey the cellular diversity of NPC, full-length scRNA-seq was performed on 1476 cells from 3 EBV+ NPC samples, including matched primary tumors and lymph node metastases. 3' droplet-based scRNA-seq (10× Genomics) was conducted on 102,525 cells from 17 EBV+ NPC and 7 non-cancerous nasopharyngeal samples (Fig. 1a) . All NPC biopsies were histologically examined by H&E and EBERs staining. NPC36 was studied using both scRNA-seq approaches to validate reproducibility. Quality checks of detected unique molecular identifier (UMI)/gene numbers and batch effects were performed.
Overall, the transcriptomes of 9 major cell types were captured according to the expression of canonical gene markers (Fig. 1b) in the 10× Genomics dataset. These cells included T cells, B cells, myeloid cells, mast cells, fibroblasts, endothelial cells (ECs), plasmacytoid dendritic cells (pDCs) and so on, which was consistent with the multiplex immunohistochemistry (multi-IHC) results (Fig. 1c). The cell clustering derived from tumors and nonmalignant tissues exhibited obvious differences, as exemplified by T cells, myeloid cells and fibroblasts (Fig. 1d). Using graph-based clustering, all cells were categorized into 19 clusters. Each of the 19 clusters harbored differentially expressed genes (DEGs) representing distinct cell types or subtypes. To distinguish malignant from nonmalignant cells, large-scale copy number variations (CNVs) were inferred based on scRNA-seq data. Putative malignant cells were grouped with extensive CNVs across the whole genome (Fig. 1e). These inferred CNVs were highly consistent with those generated from paired bulk whole-genome sequencing, such as chromosome deletions (3p, 9p, 14q, and 16q) and copy number gains (11q and 12p) (Fig. 1e), which is consistent with previous reports.
Among the 3 cases with full-length scRNA-seq data, EBV-derived sequencing reads were detected primarily located in the 0-6 kb and 150-170 kb regions of the EBV genome, covering virus latent genes such as LMP1, LMP2A/2B, and BARTs (BARF0, A73, and RPMS1), as well as lytic genes, including LF1, LF2, and BALF3 (Fig. 1f). Interestingly, typical latent and lytic genes of EBV were co-expressed within single host cells. Furthermore, potential host-virus cross-talk in malignant cells at single-cell resolution. The expression of EBV genes BNLF2a/2b was highly correlated with host genes associated with the immune response, including C3 and CFH in NPC16, OASL and ISG20 in NPC17, and IFTM1 and SERPING1 in NPC36.
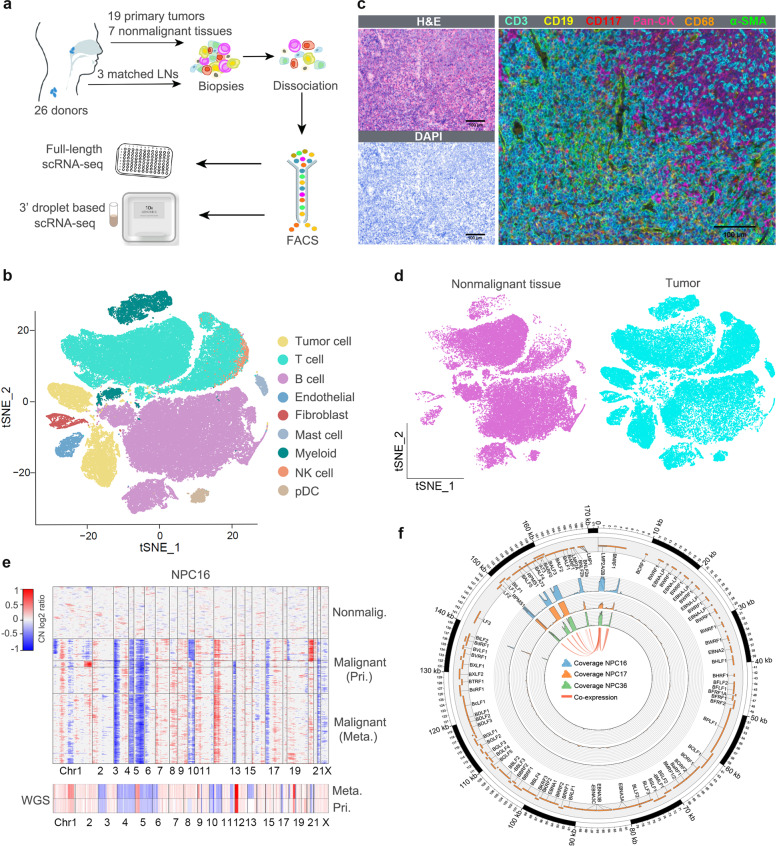 Fig.
1 Expression profiling of ~104,000 single cells from 26 samples. (Jin S, et al, 2020)
Fig.
1 Expression profiling of ~104,000 single cells from 26 samples. (Jin S, et al, 2020)
Radiation-Resistant NPC Cells Show a Metabolic Signature of Active FAO
A metabolomics approach was used to profile the global metabolic changes of radiation-responsive (CNE2) and radiation-resistant (CNE2-IR) NPC cells. The contents of 260 biochemical compounds were quantified (Fig. 2A), and obvious differences between the metabolic profiles of CNE2 and CNE2-IR cells. Relative to CNE2 cells, 130 biochemical factors were altered in CNE2-IR, and the general changes are shown as a pie chart (Fig. 2B). Among these changes, 54 metabolic factors belong to lipid metabolism (Fig. 2C). The phosphoglycerides degradation products, glycerol 3-phosphate, glycerol phosphorylcholine and multiple lysolipids, as well as the membrane sphingolipid metabolites, palmitoyl sphingomyelin and stearoyl sphingomyelin, were elevated in CNE2-IR cells. These observations may reflect an increased lipid turnover or active lipid breakdown in CNE2-IR cells.
More importantly, several acylcarnitine levels are substantially higher in CNE2-IR cells (Fig. 2D). During the oxidation of fatty acids, carnitine acts as an acyl-CoA carrier facilitating the movement of fatty acids into the matrices of the mitochondria. To further explore the utilization of fatty acids through the carnitine shuttle system, a 13C isotopomer tracing experiment was undertaken. Cells were incubated with uniformly labeled 13C16-palmitate, and the abundance of 13C-labeled palmitoyl-carnitine (C16), myristoyl-carnitine (C14), and lauroyl-carnitine (C12) was measured by ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS; Fig. 2E). 13C enrichment in acylcarnitines was higher in CNE2-IR and HK1-IR cells in response to a dose of 4 Gy radiation, indicating that radiation-resistant cells utilize palmitate and may have active fatty acid oxidation (FAO). To determine the activity of FAO, the palmitate (PA)-based oxygen consumption rate (OCR) was examined. Following radiation treatment, an obvious increase in OCR was observed in radiation-resistant cells after PA stimulation, which was then inhibited by ethyl 2-[6-(4-chlorophenoxy) hexyl] oxirane-2-carboxylate (Etomoxir, ETO), a specific FAO inhibitor (Fig. 2F). In contrast, neither PA nor ETO treatment affected the OCR of radiation-responsive cells. These results in combination with the metabolomics analysis indicate an enhanced lipid turnover and FAO in radiation-resistant NPC cells, which suggests that lipid reprogramming might play a crucial role in mediating radiation resistance.
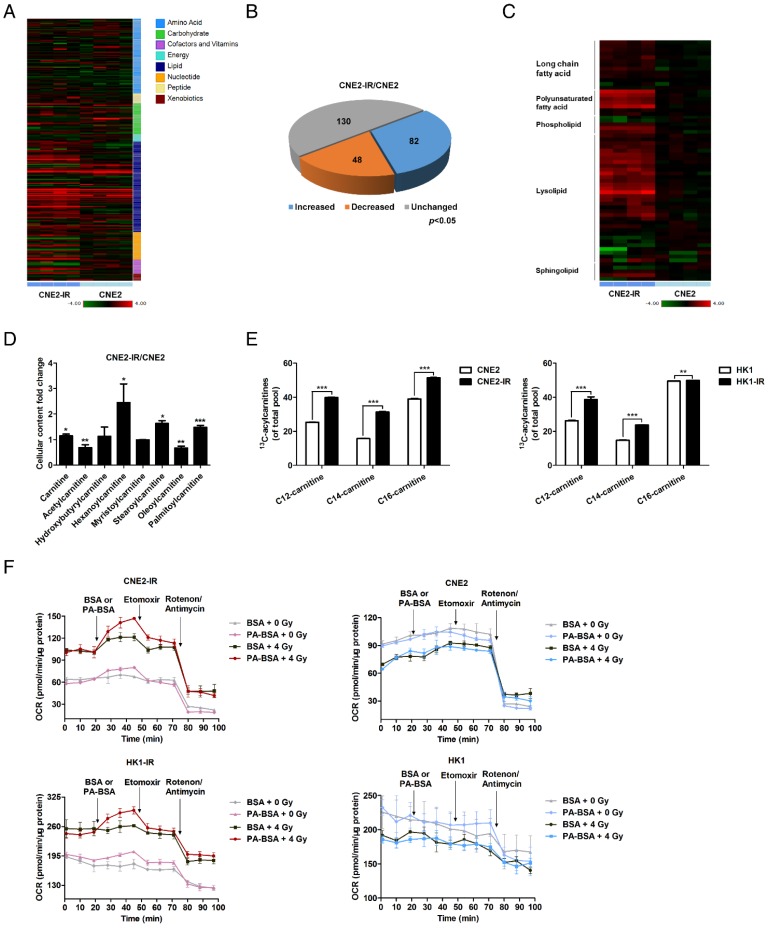 Fig.
2 Lipid turnover and fatty acid oxidation are enhanced in radiation-resistant NPC cells. (Tan Z,
et al., 2016)
Fig.
2 Lipid turnover and fatty acid oxidation are enhanced in radiation-resistant NPC cells. (Tan Z,
et al., 2016)
Nasopharyngeal tumor cells differ from normal cells in that they exhibit uncontrolled growth, genetic and epigenetic alterations, resistance to apoptosis, and the ability to invade surrounding tissues and metastasize. They may also express different biomarkers and have altered signaling pathways compared to normal nasopharyngeal cells.
The EBV is closely associated with the development of NPC. EBV infection can lead to the insertion of viral genes into the host genome, disrupt normal cell functions, and contribute to the transformation of nasopharyngeal cells into tumor cells.
Nasopharyngeal tumor cells are used to identify potential therapeutic targets and to test the efficacy of new drugs and treatments in vitro and in vivo. They are also instrumental in studying resistance mechanisms and developing personalized medicine strategies based on the genetic makeup of the tumor cells.
Filters Clear all filters
Species
- African clawed frog (1)
- American mink (1)
- Asian tiger mosquito (1)
- Atlantic salmon (1)
- Bluegill (2)
- Bluestriped grunt (1)
- Bovine (7)
- Brazilian free-tailed bat (1)
- Brown bullhead (2)
- Cabbage looper (1)
- Cabbage moth (6)
- Cat (4)
- Central mudminnow (1)
- Chicken (3)
- Chinese hamster (5)
- Chinook salmon (2)
- Chum salmon (1)
- Coho salmon (1)
- Common carp (2)
- Cotton-top tamarin (1)
- Dog (2)
- Fall armyworm (3)
- Fathead minnow (2)
- Fruit fly (1)
- Gilthead sea bream (2)
- Golden hamster (7)
- Goldfish (6)
- Gray dwarf hamster (1)
- Green monkey (2)
- Gypsy moth (1)
- Horse (1)
- Human (998)
- Japanese eel (1)
- Japanese rice fish (7)
- Koi carp (1)
- Mouse (315)
- Mouse x Gray dwarf hamster (1)
- Mouse x Rat (20)
- Northern pike (1)
- Pig (3)
- Rabbit (2)
- Rainbow trout (3)
- Rat (115)
- Rhesus macaque (1)
- Salt marsh moth (1)
- Sheep (2)
- Snakehead murrel (2)
- Sockeye salmon (1)
- Vervet monkey (2)
- Zebrafish (2)
Source
- Abdomen (1)
- Abdomen Metastasis (2)
- Adipose (2)
- Adrenal Gland (8)
- Adrenal Gland Metastasis (2)
- Aorta (4)
- Artery (1)
- Ascites (28)
- Ascites Metastasis (37)
- Bile Duct (3)
- Bladder (25)
- Bladder Metastasis (1)
- Blastocyst (1)
- Blastula (1)
- Blood (127)
- Bone (27)
- Bone Marrow (57)
- Bone Marrow Metastasis (18)
- Bone Metastasis (6)
- Brain (55)
- Brain Metastasis (8)
- Breast (30)
- Bronchus (1)
- Caudal Peduncle (1)
- Caudal Trunk (2)
- Cecum (3)
- Cerebrospinal Fluid (1)
- Cerebrospinal Fluid Metastasis (1)
- Cervix (32)
- Colon (90)
- Connective Tissue (7)
- Cornea (3)
- Cutaneous Metastasis (1)
- Dermis (2)
- Duodenum (1)
- Embryo (29)
- Endometrium (17)
- Esophagus (44)
- Eye (12)
- Eye Socket (5)
- Fetus (3)
- Fin (9)
- Foreskin (4)
- Gallbladder (1)
- Gingiva (2)
- Globe (2)
- Glomerulus (2)
- Groin (1)
- Head Kidney (2)
- Heart (4)
- Hemolymph (1)
- Hypodermis Metastasis (5)
- Ileum (1)
- Intestine (94)
- Jejunum (1)
- kidney (1)
- Kidney (27)
- Liver (35)
- Liver Metastasis (17)
- Lung (58)
- Lung Metastasis (8)
- Lymph Node (7)
- Lymph Node Metastasis (59)
- Muscle (7)
- Muscle Metastasis (2)
- Nose (2)
- Omentum Metastasis (2)
- Oral Cavity (10)
- Ovary (21)
- Ovary Metastasis (2)
- Pancreas (19)
- Pelvic Wall Metastasis (1)
- Pelvis (1)
- Perianal Space Metastasis (1)
- Pericardial Effusion (1)
- Pericardial Effusion Metastasis (2)
- Perineus (1)
- Peripheral Blood (126)
- Peripheral Nervous System (21)
- Peritoneal Effusion (2)
- Peritoneum (1)
- Peritoneum Metastasis (1)
- Pharynx (3)
- Pituitary Gland (7)
- Pleural Effusion (54)
- Pleural Effusion Metastasis (46)
- Prostate (7)
- Rectum (15)
- Renal Pelvis (1)
- Retroperitoneal Space (2)
- Salivary Gland (2)
- Skeletal Muscle (5)
- Skin (32)
- Skin Metastasis (3)
- Small Intestine (4)
- Small Intestine Metastasis (1)
- Smooth Muscle (2)
- Soft Tissue (1)
- Soft Tissue Metastasis (1)
- Spinal Cord (2)
- Stomach (4)
- Testis (15)
- Thoracic Cavity Metastasis (6)
- Thymus (5)
- Thyroid Gland (16)
- Thyroid Gland Metastasis (1)
- Tongue (5)
- Trachea (1)
- Umbilical Cord (1)
- Umbilical Cord Blood (1)
- Urachus (1)
- Ureter (1)
- Uterus (54)
- Uvea (2)
- Vagina (2)
- Vulva (1)
Disease
- Acute Biphenotypic Leukemia (1)
- Acute Erythroid Leukemia (4)
- Acute Megakaryoblastic Leukemia (4)
- Acute Monocytic Leukemia (9)
- Acute Myeloid Leukemia (25)
- Acute Promyelocytic Leukemia (2)
- Adrenal Gland Neuroblastoma (11)
- Adult B Acute Lymphoblastic leukemia (1)
- Adult B Acute Lymphoblastic Leukemia (6)
- Adult T Acute Lymphoblastic Leukemia (6)
- Adult T Lymphoblastic Lymphoma (2)
- Adult T-Cell Leukemia/Lymphoma (1)
- Alveolar Rhabdomyosarcoma (4)
- Alveolar Ridge Squamous Cell Carcinoma (1)
- Amelanotic Melanoma (3)
- Ampulla of Vater Adenocarcinoma (1)
- Ampulla of Vater Adenosquamous Carcinoma (3)
- Anaplastic Astrocytoma (3)
- Anaplastic Large Cell Lymphoma (7)
- Askin Tumor (1)
- Astrocytoma (5)
- B Acute Lymphoblastic Leukemia (2)
- B-Cell Non-Hodgkin Lymphoma (5)
- Bare Lymphocyte Syndrome Type 2 (1)
- Barrett Adenocarcinoma (2)
- Benign Prostatic Hyperplasia (1)
- Bladder Carcinoma (14)
- Bladder Squamous Cell Carcinoma (1)
- Bovine Leukemia (2)
- Breast Adenocarcinoma (4)
- Breast Carcinoma (9)
- Breast Ductal Carcinoma (2)
- Burkitt Lymphoma (17)
- Canavan Disease (1)
- Canine Histiocytic Sarcoma (1)
- Cecum Adenocarcinoma (3)
- Central Nervous System Lymphoma (2)
- Cervical Adenocarcinoma (2)
- Cervical Adenosquamous Carcinoma (2)
- Cervical Small Cell Carcinoma (1)
- Cervical Squamous Cell Carcinoma (2)
- Chicken Bursal Lymphoma (2)
- Childhood B Acute Lymphoblastic Leukemia (13)
- Childhood T Acute Lymphoblastic Leukemia (16)
- Childhood T Lymphoblastic Lymphoma (1)
- Cholangiocarcinoma (2)
- Chronic Eosinophilic Leukemia (1)
- Chronic Lymphocytic Leukemia (2)
- Chronic Myeloid Leukemia (23)
- Clear Cell Renal Cell Carcinoma (2)
- Colon Adenocarcinoma (55)
- Colon Carcinoma (34)
- Colorectal Adenocarcinoma (1)
- Colorectal Carcinoma (1)
- Congenital Pure Red Cell Aplasia (1)
- Cutaneous Melanoma (10)
- Dedifferentiated Chondrosarcoma (1)
- Desmoplastic Melanoma (1)
- Diffuse Large B-Cell Lymphoma (28)
- Down Syndrome (2)
- EBV-Related Burkitt Lymphoma (12)
- Embryonal Carcinoma (3)
- Embryonal Rhabdomyosarcoma (3)
- Endometrial Adenocarcinoma (13)
- Endometrial Adenosquamous Carcinoma (2)
- Endometrial Carcinoma (2)
- Endometrioid Stromal Sarcoma (1)
- Epithelioid Hemangioendothelioma (1)
- Epithelioid Sarcoma (3)
- Esophageal Adenocarcinoma (6)
- Esophageal Squamous Cell Carcinoma (41)
- Essential Thrombocythemia (1)
- Ewing Sarcoma (2)
- Extraskeletal Myxoid Chondrosarcoma (1)
- Fanconi Anemia (1)
- Fibrosarcoma (1)
- Follicular Lymphoma (2)
- Gallbladder Carcinoma (2)
- Gallbladder Undifferentiated Carcinoma (2)
- Gastric Adenocarcinoma (6)
- Gastric Adenosquamous Carcinoma (1)
- Gastric Carcinoma (5)
- Gastric Choriocarcinoma (1)
- Gastric Fundus Carcinoma (1)
- Gastric Signet Ring Cell Adenocarcinoma (1)
- Gastric Small Cell Carcinoma (2)
- Gastric Tubular Adenocarcinoma (5)
- Gastroesophageal Junction Adenocarcinoma (1)
- Gestational Choriocarcinoma (1)
- Gingival Squamous Cell Carcinoma (2)
- Glioblastoma (18)
- Gliosarcoma (1)
- Goldfish Erythrophoroma (4)
- Hairy Cell Leukemia (1)
- Hamster Kidney Tumor (1)
- Hamster Pancreatic Ductal Adenocarcinoma (1)
- Hamster Uterine Leiomyosarcoma (1)
- Hepatoblastoma (2)
- Hepatocellular Carcinoma (6)
- Hepatosplenic T-Cell Lymphoma (2)
- Hereditary Thyroid Gland Medullary Carcinoma (1)
- High Grade B-Cell Lymphoma (1)
- High Grade Ovarian Serous Adenocarcinoma (8)
- Hodgkin Lymphoma (9)
- Hypopharyngeal Squamous Cell Carcinoma (2)
- Infectious Mononucleosis (1)
- Intrahepatic Cholangiocarcinoma (6)
- Invasive Breast Carcinoma of No Special Type (12)
- Invasive Breast Lobular Carcinoma (1)
- Kidney Neoplasm (1)
- Kidney Rhabdoid Tumor (1)
- Krukenberg Tumor (1)
- Liposarcoma (1)
- Lung Adenocarcinoma (17)
- Lung Giant Cell Carcinoma (8)
- Lung Large Cell Carcinoma (9)
- Lung Mucoepidermoid Carcinoma (1)
- Lung Non-Small Cell Carcinoma (2)
- Lung Small Cell Carcinoma (25)
- Lung Squamous Cell Carcinoma (9)
- Lymphoblastic Lymphoma (1)
- Malignant Peripheral Nerve Sheath Tumor (1)
- Mantle Cell Lymphoma (5)
- Mature Gastric Teratoma (1)
- Maxillary Sinus Squamous Cell Carcinoma (1)
- Medaka Hepatoma (2)
- Medulloblastoma (3)
- Melanoma (24)
- Meningioma (2)
- Minimally Invasive Lung Adenocarcinoma (1)
- Monophasic Synovial Sarcoma (1)
- Mouse Bladder Transitional Cell Carcinoma (1)
- Mouse Chondrosarcoma (1)
- Mouse Colon Adenocarcinoma (3)
- Mouse Ependymoma (2)
- Mouse Erythroid Leukemia (13)
- Mouse Fibrosarcoma (5)
- Mouse Glioblastoma (1)
- Mouse Hemangioendothelioma (1)
- Mouse Hepatocellular Carcinoma (1)
- Mouse Insulinoma (3)
- Mouse Intestinal Tract Neuroendocrine Adenoma (1)
- Mouse Islet Cell Adenoma (1)
- Mouse Kidney Carcinoma (1)
- Mouse Leukemia (10)
- Mouse Leydig Cell Tumor (1)
- Mouse Lymphoma (8)
- Mouse Mammary Gland Malignant Neoplasm (23)
- Mouse Melanoma (9)
- Mouse Multiple Myeloma (5)
- Mouse Myeloid Leukemia (3)
- Mouse Neoplasm (1)
- Mouse Neuroblastoma (21)
- Mouse Oral Cavity Squamous Cell Carcinoma (1)
- Mouse Osteosarcoma (3)
- Mouse Pituitary Gland Neoplasm (1)
- Mouse Plasmacytoma (1)
- Mouse Precursor T Cell Lymphoblastic Lymphoma/Leukemia (2)
- Mouse Pulmonary Adenoma (1)
- Mouse Pulmonary Malignant Tumor (3)
- Mouse Pulmonary Squamous Cell Carcinoma (1)
- Mouse Rectum Carcinoma (2)
- Mouse Reticulum Cell Sarcoma (2)
- Mouse Sarcoma (1)
- Mouse Teratocarcinoma (8)
- Mouse Thymic Lymphoma (3)
- Mycosis Fungoides (1)
- Myelodysplastic Syndrome (1)
- Myxofibrosarcoma (1)
- Natural Killer Cell Lymphoblastic Leukemia/Lymphoma (2)
- Neuroblastoma (26)
- Oral Cavity Squamous Cell Carcinoma (15)
- Osteoid Osteoma (1)
- Osteosarcoma (15)
- Ovarian Carcinoma (1)
- Ovarian Clear Cell Adenocarcinoma (1)
- Ovarian Endometrioid Adenocarcinoma (4)
- Ovarian Granulosa Cell Tumor (1)
- Ovarian Mucinous Adenocarcinoma (2)
- Ovarian Serous Adenocarcinoma (2)
- Ovarian Serous Cystadenocarcinoma (2)
- Ovarian Small Cell Carcinoma (1)
- Pancreatic Adenocarcinoma (13)
- Pancreatic Carcinoma (5)
- Pancreatic Ductal Adenocarcinoma (12)
- Papillomavirus-Independent Cervical Squamous Cell Carcinoma (1)
- Papillomavirus-Related Cervical Adenocarcinoma (7)
- Papillomavirus-Related Cervical Squamous Cell Carcinoma (4)
- Papillomavirus-Related Endocervical Adenocarcinoma (16)
- Paroxysmal Nocturnal Hemoglobinuria (3)
- Pharyngeal Squamous Cell Carcinoma (1)
- Plasma Cell Myeloma (15)
- Pleural Epithelioid Mesothelioma (5)
- Pleural Sarcomatoid Mesothelioma (2)
- Poorly Differentiated Thyroid Gland Carcinoma (1)
- Primary Cutaneous T-Cell Non-Hodgkin Lymphoma (1)
- Primary Effusion Lymphoma (7)
- Primitive Neuroectodermal Tumor (1)
- Prostate carcinoma (1)
- Prostate Carcinoma (9)
- Rat C-Cell Carcinoma (1)
- Rat Cholangiocarcinoma (1)
- Rat Colon Adenocarcinoma (5)
- Rat Digestive System Neoplasm (1)
- Rat Fibrosarcoma (1)
- Rat Hepatocellular Carcinoma (20)
- Rat Histiocytic Sarcoma (1)
- Rat Insulinoma (2)
- Rat Leukemia (1)
- Rat Leydig Cell Adenoma (1)
- Rat Lung Carcinoma (1)
- Rat Malignant Glioma (4)
- Rat Malignant Meningioma (1)
- Rat Malignant Oligodendroglioma (2)
- Rat Malignant Thymoma (3)
- Rat Mammary Gland Adenocarcinoma (10)
- Rat Neuroblastoma (3)
- Rat Osteosarcoma (2)
- Rat Pituitary Gland Neoplasm (6)
- Rat Prostate Adenocarcinoma (3)
- Rat Rhabdomyosarcoma (1)
- Rat Sarcoma (2)
- Rat Squamous Cell Carcinoma (1)
- Rat Urinary Bladder Transitional Cell Carcinoma (2)
- Rat Urinary System Neoplasm (6)
- Rectal Adenocarcinoma (13)
- Rectosigmoid Adenocarcinoma (1)
- Recurrent Bladder Carcinoma (1)
- Renal Cell Carcinoma (7)
- Renal Pelvis Urothelial Carcinoma (1)
- Retinoblastoma (11)
- Sacral Chordoma (1)
- Sacrococcygeal Teratoma (1)
- Salivary Gland Squamous Cell Carcinoma (1)
- Sezary Syndrome (1)
- Shwachman-Diamond Syndrome (1)
- Skin Squamous Cell Carcinoma (2)
- Splenic Marginal Zone Lymphoma (1)
- Testicular Embryonal Carcinoma (8)
- Testicular Teratoma (2)
- Testicular Yolk Sac Tumor (1)
- Thyroid Gland Anaplastic Carcinoma (10)
- Thyroid Gland Follicular Carcinoma (4)
- Thyroid Gland Papillary Carcinoma (3)
- Thyroid Gland Sarcoma (1)
- Thyroid Gland Squamous Cell Carcinoma (2)
- Tongue Adenosquamous Carcinoma (1)
- Tongue Squamous Cell Carcinoma (6)
- Type I Endometrial Adenocarcinoma (1)
- Ureter Urothelial Carcinoma (1)
- Uterine Carcinosarcoma (2)
- Uterine Corpus Leiomyosarcoma (1)
- Uterine Corpus Sarcoma (2)
- Uveal Melanoma (2)
- Vaginal Melanoma (2)
- Vulvar Melanoma (1)
- Vulvar Squamous Cell Carcinoma (1)
Description: The Paclitaxel-resistant cell line WSU-HN30/Taxol has been developed by repeatedly exposing the ...
Description: Japanese maxillary simus squamous carcinoma.
Description: The Cisplatin-resistant cell line FADU/DDP has been developed by repeatedly exposing the parent ...