Colon Ascendens Stent Peritonitis (CASP)-Induced Sepsis Model
Creative Bioarray offers specialized expertise and extensive experience in the pharmaceutical industry to aid our clients in assessing the therapeutic potential of test compounds for various immunological diseases, such as sepsis. We are equipped to provide a colon ascendens stent peritonitis (CASP)-induced sepsis model. With our wealth of experience and knowledge, we can tailor custom study designs to specifically address your unique requirements.
CASP model involves the placement of a stent within the ascending colon of the animal, allowing for continuous leakage of feces into the peritoneum and subsequent development of sepsis. This model closely mimics the shock and organ dysfunction observed in clinical sepsis cases. The CASP model has demonstrated the induction of acute lung and kidney injuries, along with bone marrow cell dysfunction. The severity of the disease can be modulated based on the size of the stent used. While this model may not fully recapitulate the hemodynamic features of clinical sepsis, it does exhibit both severe inflammatory response syndrome (SIRS) and complementary anti-inflammatory response (CARS) simultaneously, as evidenced by the rapid elevation of both pro-inflammatory and anti-inflammatory cytokines. The CASP model closely resembles the systemic inflammation observed in clinical sepsis, accompanied by multiple organ failure, particularly in the lung, liver, and kidney. Therefore, the CASP model stands as a crucial and relevant tool for advancing our understanding of sepsis and developing potential therapeutic interventions.
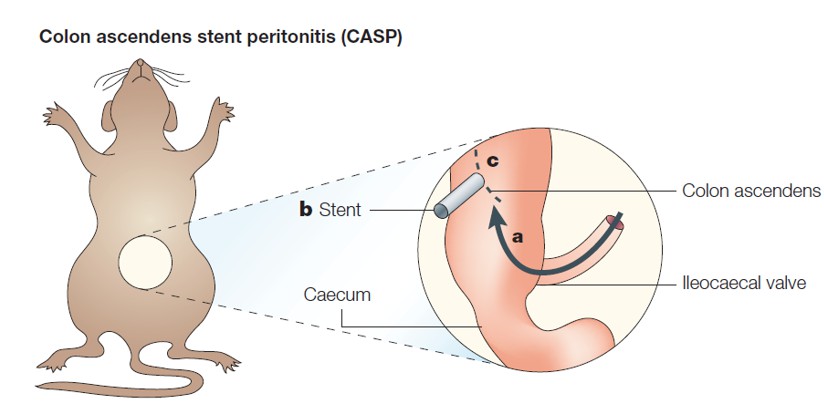 Fig. 1 CASP model of sepsis
Fig. 1 CASP model of sepsis
Our Colon Ascendens Stent Peritonitis (CASP)-Induced Sepsis Model
- Available Animal
Rat
Mouse - Modeling Method
Step 1: After anesthetization, animals are secured on an operating table, and undergo hair removal and disinfection.
Step 2: A longitudinal incision is made in the appropriate area, followed by sequential incisions in the cortical and muscle layers to expose the caecum and colon ascendens.
Step 3: After identifying the colon ascendens and the cecum, a plastic stent is inserted into the antimesenteric wall of the cecum 1.0 cm distal to the ileocecal valve.
Step 4: Stool is milked out and fluid resuscitation is performed via intraperitoneal administration with fluid solution.
Step 5: The caecum is repositioned within the abdominal cavity, and the muscle and skin layers are sequentially sutured using a surgical line. - Endpoints
- Survival rate
- Clinical observation
- Cytokine analysis: IL-1, TNF-α, IL-6, IFN-γ, etc.
- Histology analysis: H&E staining
- Serum analysis: creatinine kinase, BUN, ALT, AST, etc.
- qPCR or Western Blot
- Other customized endpoints: available upon request.
Example Data
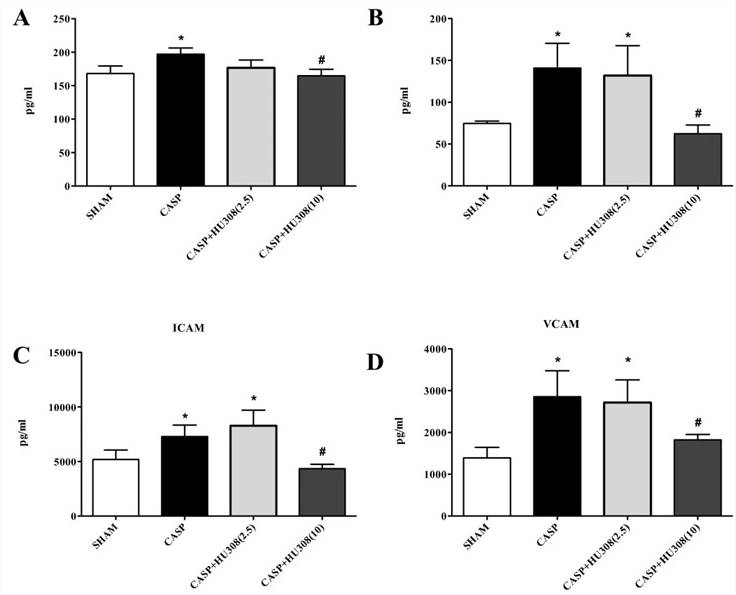 Fig. 2 Colon ascendens stent peritonitis-induced sepsis - inflammatory mediators (pg/ml). (A) TNFα, (B) IL-1β, (C) intercellular adhesion molecule (ICAM), (D) vascular cell adhesion molecule (VCAM). SHAM, control group (n = 9); colon ascendens stent peritonitis (CASP), sepsis group (n = 9); CASP + HU308 (2.5/10 mg/kg), CASP plus cannabinoid receptor 2 agonist (2.5 or 10 mg/kg HU308; n = 9).
Fig. 2 Colon ascendens stent peritonitis-induced sepsis - inflammatory mediators (pg/ml). (A) TNFα, (B) IL-1β, (C) intercellular adhesion molecule (ICAM), (D) vascular cell adhesion molecule (VCAM). SHAM, control group (n = 9); colon ascendens stent peritonitis (CASP), sepsis group (n = 9); CASP + HU308 (2.5/10 mg/kg), CASP plus cannabinoid receptor 2 agonist (2.5 or 10 mg/kg HU308; n = 9).
Furthermore, we also provide other sepsis models that maybe you are interested in:
Quotation and Ordering
Creative Bioarray is a Contract Research Organization (CRO) that specializes in conducting in vivo efficacy studies. With extensive experience in this field, our scientists have developed a wide range of disease models to assist clients in expediting their drug development processes. If you are interested in our services, please feel free to contact us or submit an inquiry to us directly.
References
- Buras, J., et al. Animal Models of sepsis: setting the stage. Nat Rev Drug Discov, 2005, 4: 854-865.
- Lehmann, C., et al. Cannabinoid receptor 2 activation reduces intestinal leukocyte recruitment and systemic inflammatory mediator release in acute experimental sepsis. Critical care, 2012, 16: 1-11.