- You are here: Home
- Disease Models
- Inflammation & Autoimmune Disease Models
- Scleroderma Model
Disease Models
- Oncology Models
-
Inflammation & Autoimmune Disease Models
- Rheumatoid Arthritis Models
- Glomerulonephritis Models
- Multiple Sclerosis (MS) Models
- Ocular Inflammation Models
- Sjögren's Syndrome Model
- LPS-induced Acute Lung Injury Model
- Peritonitis Models
- Passive Cutaneous Anaphylaxis Model
- Delayed-Type Hypersensitivity (DTH) Models
- Inflammatory Bowel Disease Models
- Systemic Lupus Erythematosus Animal Models
- Asthma Model
- Sepsis Model
- Psoriasis Model
- Atopic Dermatitis (AD) Model
- Scleroderma Model
- Gouty Arthritis Model
- Carrageenan-Induced Air Pouch Synovitis Model
- Carrageenan-Induced Paw Edema Model
- Experimental Autoimmune Myasthenia Gravis (EAMG) Model
-
Cardiovascular Disease Models
- Surgical Models
- Animal Models of Hypertension
- Venous Thrombosis Model
- Atherosclerosis model
- Cardiac Arrhythmia Model
- Hyperlipoidemia Model
- Doxorubicin-induced Heart Failure Model
- Isoproterenol-induced Heart Failure Model
- Arterial Thrombosis Model
- Pulmonary Arterial Hypertension (PAH) Models
- Heart Failure with Preserved Ejection Fraction (HFpEF) Model
-
Neurological Disease Models
- Alzheimer's Disease Modeling and Assays
- Seizure Models
- Parkinson's Disease Models
- Ischemic Stroke Models
- Acute Spinal Cord Injury (ASCI) Model
- Traumatic Brain Injury (TBI) Model
- Hypoxic-Ischemic Encephalopathy (HIE) Model
- Tourette Syndrome (TS) Model
- Amyotrophic Lateral Sclerosis (ALS) Model
- Huntington's Disease (HD) Model
- Intracerebral hemorrhage (ICH) Models
- Pain Models
- Metabolic Disease Models
- Liver Disease Models
- Rare Disease Models
- Respiratory Disease Models
- Digestive Disease Models
-
Urology Disease Models
- Cisplatin-induced Nephrotoxicity Model
- Unilateral Ureteral Obstruction Model
- 5/6 Nephrectomy Model
- Renal Ischemia-Reperfusion Injury (RIRI) Model
- Diabetic Nephropathy (DN) Models
- Passive Heymann Nephritis (PHN) Model
- Adenine-Induced Chronic Kidney Disease (CKD) Model
- Kidney Stone Model
- Doxorubicin-Induced Nephropathy Model
- Orthopedic Disease Models
- Ocular Disease Models
- Skin Disease Models
- Infectious Disease Models
Scleroderma Model
At Creative Bioarray, we boast a wealth of experience in scleroderma research. Our team will collaborate closely with you, guiding you through the entire process from selecting the most suitable models, and designing a meticulous study plan, to the final data analysis and reporting. We are committed to providing clients worldwide with the most comprehensive preclinical animal testing services tailored specifically for scleroderma. This includes developing validated animal models of scleroderma and meticulously evaluating the toxicity and efficacy of drug candidates in rodents, among other services. With our expertise and dedication, you can be assured of obtaining reliable and actionable insights that will propel your scleroderma research forward.
Scleroderma, or systemic sclerosis (SSc), is an immune-mediated chronic disorder with a systemic involvement characterized by small vessel alterations and progressive fibrosis of the skin and internal organs, such as lungs, gastrointestinal tract, and heart. SSc is a devastating and severe disease, burdened with significant morbidity and mortality. When a severe pulmonary or cardiac involvement is present, patients with SSc have a 3-year survival rate of 47–56%. Despite recent advances, however, an effective disease-modifying treatment approved for the treatment of SSc is currently lacking.
The precise etiopathogenesis of SSc remains enigmatic. Similar to numerous other immune-mediated conditions, the prevailing hypothesis suggests that a confluence of predisposing genetic factors and an inciting stimulus or occurrence leads to a breakdown in self-tolerance, resulting in chronic immune system activation. This activation is not promptly modulated by endogenous regulatory cells and checkpoints, leading to unchecked immune response. It is postulated that the microvessel wall serves as the initial locus of this persistent immune-mediated inflammation, triggering alterations that encompass not merely the endothelium, but all layers of the vessel wall. These alterations are followed by myofibroblast activation, excessive deposition of extracellular matrix (ECM), and unchecked tissue fibrosis.
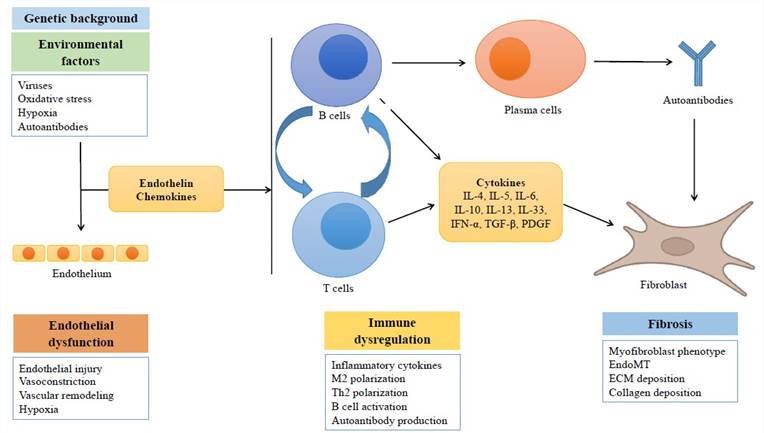 Fig. 1 Simplified scheme showing the main processes involved in the pathogenesis of systemic sclerosis
Fig. 1 Simplified scheme showing the main processes involved in the pathogenesis of systemic sclerosis
Our Scleroderma Model
- Available Animal
Mouse - Modeling Method
Mice are subcutaneously injected with bleomycin in the central area of the back once a day for several weeks. - Endpoints
- Clinical observation
- Histology analysis (skin and lung): H&E staining, Masson staining, Toluidine blue staining
- Body weight
- Skin thickness
- Hydroxyproline analysis
- Other customized endpoints: available upon request
Example Data
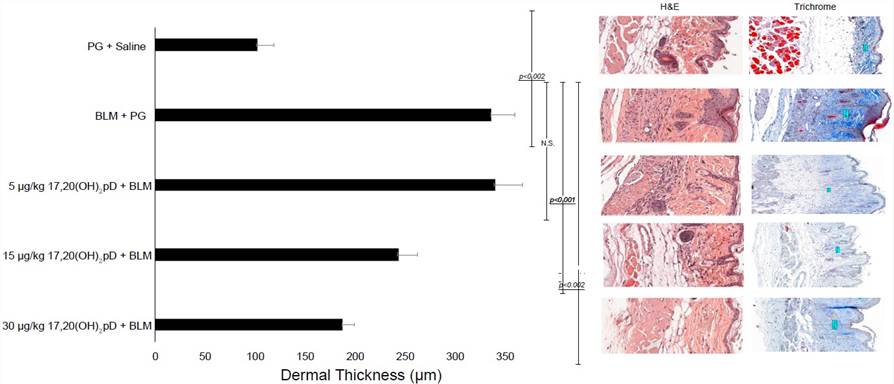 Fig. 2 17,20S(OH)2pD decreases dermal thickness in BLM model of fibrosis. Left panel: quantification of the dermal thickness; middle panel: H&E stained sections; right panel: Trichrome stained sections.
Fig. 2 17,20S(OH)2pD decreases dermal thickness in BLM model of fibrosis. Left panel: quantification of the dermal thickness; middle panel: H&E stained sections; right panel: Trichrome stained sections.
Quotation and Ordering
Creative Bioarray is committed to sharing our groundbreaking technologies and vast expertise with our esteemed clients, aiming to amplify the significance and impact of their remarkable research efforts. If you are interested in our services, please feel free to contact us at any time or submit an inquiry to us directly.
References
- Brown Lobbins M L, et al. 17, 20S (OH) 2pD Can Prevent the Development of Skin Fibrosis in the Bleomycin-Induced Scleroderma Mouse Model. International Journal of Molecular Sciences, 2021, 22(16): 8926.
- Benfaremo, D., et al. Systemic sclerosis: from pathophysiology to novel therapeutic approaches. Biomedicines, 2022, 10(1): 163.
For research use only. Not for any other purpose.

