Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

Testicular Tumor Cells
- Product List
- Background
- Applications
- Scientific Data
Testicular tumors are a relatively rare yet significant subset of cancers that predominantly affect young men. Testicular tumors can arise from different cell types within the testes, including germ cells, which give rise to sperm, and non-germ cells, which comprise the supportive tissue. The diversity of cell origins contributes to the varied nature of testicular tumors and their distinct pathologies.
Testicular Tumor Cell Characteristics
- Cellular composition and types. Testicular tumors primarily originate from germ cells, which are responsible for sperm production. The two main categories of germ cell tumors are seminomas and non-seminomas. Seminomas are characterized by their slow growth and better prognosis, while non-seminomas, which include embryonal carcinoma, yolk sac tumors, and choriocarcinoma, are generally more aggressive.
- Genetic and molecular features. Testicular tumor cells exhibit distinct genetic alterations that contribute to their malignancy. Common chromosomal abnormalities include the isochromosome 12p, which is found in approximately 80% of germ cell tumors. This genetic hallmark can serve as a biomarker for diagnosis and monitoring. Additionally, mutations in genes such as KIT and TP53 have been implicated in tumor progression, highlighting potential targets for therapeutic intervention.
- Tumor microenvironment. The tumor microenvironment plays a critical role in the behavior of testicular tumor cells. Interactions between tumor cells and surrounding stromal cells can influence tumor growth and metastasis. For instance, the presence of certain immune cells, such as macrophages, can either promote or inhibit tumor progression. Understanding these dynamics is essential for developing immunotherapeutic strategies.
Drug Screening
Researchers use testicular tumor cell lines and patient-derived xenografts to screen potential anti-cancer drugs. These models allow for the evaluation of drug efficacy and the identification of potential side effects before moving to more complex and costly clinical trials.
Targeted Therapies
The genetic and molecular profiling of testicular tumor cells has led to the identification of specific targets for targeted therapies. For instance, mutations in the KIT gene are associated with certain testicular cancers, and drugs that inhibit KIT activity have shown promise in treating these tumors.
Immunotherapy Research
The field of immunotherapy has benefited from the study of testicular tumor cells. These cells can be used to develop and test immunotherapeutic strategies that harness the patient's immune system to recognize and attack tumor cells.
Cancer Vaccine Research
Research into cancer vaccines often involves the use of testicular tumor antigens to stimulate an immune response. By exposing the immune system to these antigens, it may be possible to train it to target and destroy tumor cells throughout the body.
Autophagy Levels in Cisplatin-Resistant Testicular Cancer Cells Depend on Panx-1 Expression
Pannexin1 (Panx-1) is a gap junction channel protein that mediates the release of intracellular ATP during autophagy and thus plays an important role in tumor cell apoptosis and chemo-resistance. Cisplatin-resistant I-10 testicular cancer cell lines (I-10/CDDP) autophagy-associated proteins (p62, p-mTOR, mTOR, and LC3) exhibited high levels of autophagy in their expression, while LC3-II expression was more significantly in the presence of lysosomal degradation blocked by chloroquine (CQ).
To further explore the Panx-1-induced autophagy in I-10/DDP cells, two independent shRNAs and an expression vector encoding mouse full-length Panx-1 (mPanx-1) were transfected into I-10/DDP cells. The role of the Panx-1 channel on the level of autophagy was further elucidated via shRNA-mediated knockdown and full-length panx-1 gene overexpression. As shown in Fig. 1a, Panx-1 expression was decreased in the shRNA-transfected group compared to the NC group. In addition, Panx-1 knockdown significantly reduced extracellular ATP levels in I-10/CDDP cells (Fig. 1b). Panx-1 knockdown significantly elevated LC3II expression and also significantly decreased p62 and p-mTOR expression in I-10/DDP cells, while the presence of CQ more significantly upregulated LC3II protein expression (Fig. 1c). Furthermore, the autophagy flux of GFP-LC3B and autophagosomes were increased in the Panx-1 knockdown cells (Fig. 1d, e).
Consistent with the above, ectopic expression of Panx-1 (Fig. 2a) increased extracellular ATP levels in I-10/CDDP cells (Fig. 2b). Similarly, ectopic expression of Panx-1 significantly decreased the expression of LC3-II and increased the expression of p62 and p-mTOR, while the presence of CQ more significantly downregulated LC3II protein expression (Fig. 2c). Furthermore, the autophagy flux of GFP-LC3B and autophagosomes was also decreased in the mPanx-1 group (Fig. 2d, e). Taken together, autophagy of I-10/CDDP cells is regulated by the expression of Panx-1.
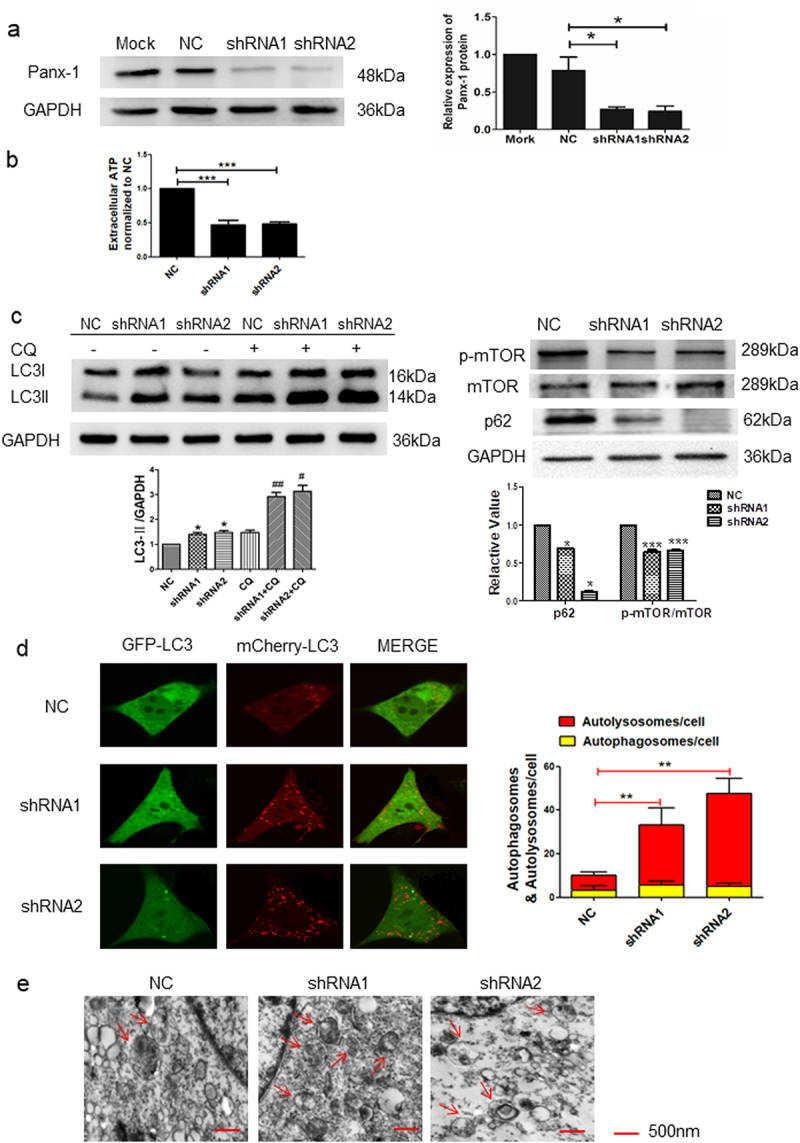 Fig. 1 Knockdown of Panx-1 increased autophagy in I-10/CDDP cells. (a) Immunoblots showing Panx-1 expression in Mock, NC, and shRNA-Panx-1 I-10/CDDP cells. (b) The extracellular ATP was assessed by Luminescence assay. (c) Western blots showed the expression of p62, p-mTOR, and mTOR proteins after the knockdown of Panx-1 and the expression of LC3 protein in the presence of CQ (10 μmol). (d) Representative fluorescence images showed autophagosomes and autolysosomes in the indicated group. (e) Representative TEM images showed autophagosomes. (Yuan M, et al, 2022)
Fig. 1 Knockdown of Panx-1 increased autophagy in I-10/CDDP cells. (a) Immunoblots showing Panx-1 expression in Mock, NC, and shRNA-Panx-1 I-10/CDDP cells. (b) The extracellular ATP was assessed by Luminescence assay. (c) Western blots showed the expression of p62, p-mTOR, and mTOR proteins after the knockdown of Panx-1 and the expression of LC3 protein in the presence of CQ (10 μmol). (d) Representative fluorescence images showed autophagosomes and autolysosomes in the indicated group. (e) Representative TEM images showed autophagosomes. (Yuan M, et al, 2022)
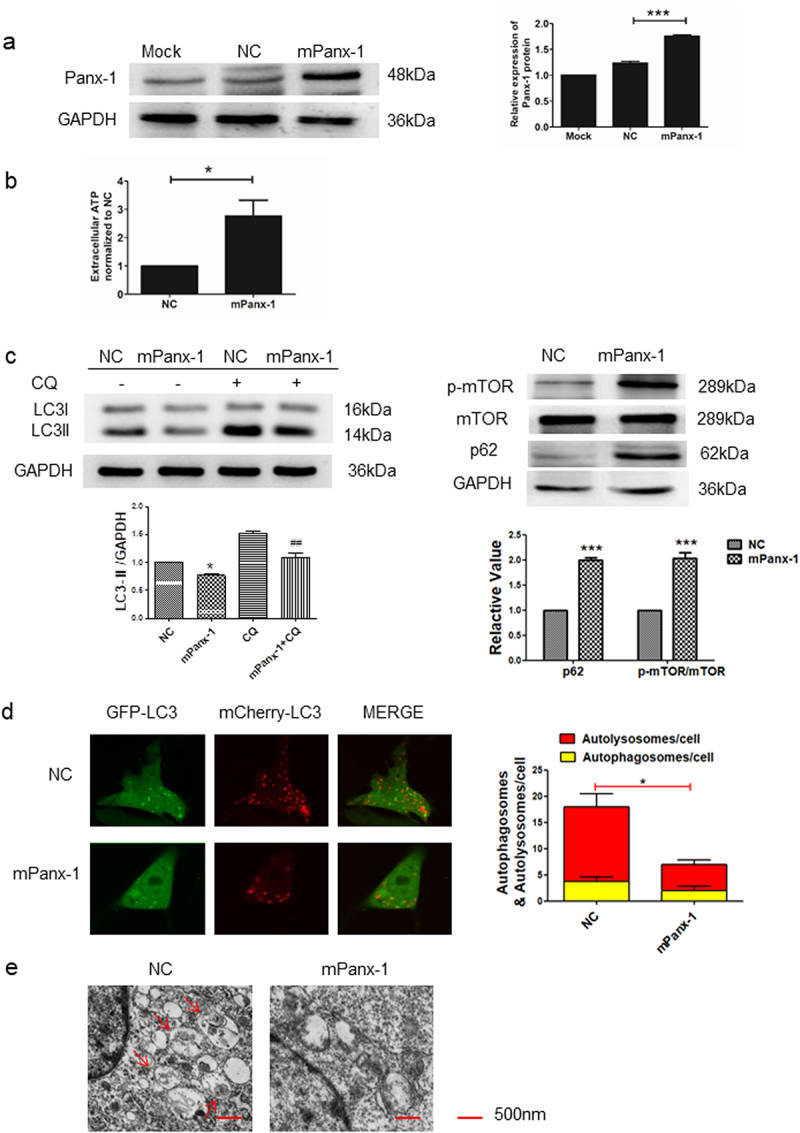 Fig. 2 Overexpression of Panx-1 decreased autophagy in I-10/CDDP cells. (a) Immunoblots showing Panx-1 expression in Mork, NC and mPanx-1 I-10/CDDP cells. (b) The extracellular ATP was assessed by luminescence assay. (c) Western blots showed the expression of p62, p-mTOR, and mTOR proteins after Panx-1 overexpression and the expression of LC3 protein in the presence of CQ (10 μmol). (d) Representative fluorescence images showed autophagosomes and autolysosomes in the indicated group. (e) Representative TEM images showed autophagosomes. (Yuan M, et al, 2022)
Fig. 2 Overexpression of Panx-1 decreased autophagy in I-10/CDDP cells. (a) Immunoblots showing Panx-1 expression in Mork, NC and mPanx-1 I-10/CDDP cells. (b) The extracellular ATP was assessed by luminescence assay. (c) Western blots showed the expression of p62, p-mTOR, and mTOR proteins after Panx-1 overexpression and the expression of LC3 protein in the presence of CQ (10 μmol). (d) Representative fluorescence images showed autophagosomes and autolysosomes in the indicated group. (e) Representative TEM images showed autophagosomes. (Yuan M, et al, 2022)
Cordycepin-Induced Apoptosis in Mouse Testicular Tumor Cells by Activating PERK/EIF2α Signaling Pathways
Studies have demonstrated that misfolded proteins could prompt ER stress to restore protein homeostasis. If stress is prolonged, apoptotic cell death succeeds. To further study whether cordycepin would regulate ER stress pathways inducing apoptosis in the mouse Leydig tumor (MA-10) cells, ER stress-related proteins, such as PERK, EIF2α, p-EIF2α, ATF3, CHOP, ATF6β, IRE1α, cleaved XBP1, and cleaved caspase-12, in cordycepin-treated MA-10 cells were analyzed by Western blotting (Fig. 3A). Results showed that expression of PERK gradually decreased by cordycepin treatments (0-100 μmol/L); however, expression of PERK rebounded by treatments of 500 and 1000 μmol/L cordycepin for 12 hours (Fig. 3B). Interestingly, expression of PERK significantly decreased by cordycepin (50-1000 μmol/L) in 24-hour treatment (Fig. 3B). Expressions of p-EIF2α were stimulated by 12- and 24-hour treatments of 500 and 1000 μmol/L cordycepin, respectively (Fig. 3B). Expression of ATF3 was stimulated by 100 μmol/L cordycepin after 12- and 24-hour treatments (Fig. 3B). The CHOP expression showed no significant change with cordycepin treatments for 12 and 24 hours (Fig. 3B).
Total ATF6β, IRE1α, cleaved XBP1plus total, and cleaved caspase-12 were also detected by Western blotting with the treatments of cordycepin (0, 10, 50, 100, 500, and 1000 μmol/L) for 12 and 24 hours. Results showed that 12- and 24-hour cordycepin treatments did not affect the expressions of ATF6β (Fig. 4A, B). Treatments with cordycepin (10-1000 μmol/L) for 12 and 24 hours significantly decreased the expressions of IRE1α (Fig. 4B). A known value of 1000 μmol/L cordycepin induced the maximal levels of XBP1 expression at 12 hours (Fig. 4B). Furthermore, 50 and 100 μmol/L cordycepin induced the expressions of cleaved caspase-12 at 12 hours (Fig. 4B). These results indicated that cordycepin could regulate ER stress pathways to induce apoptosis in MA-10 cells.
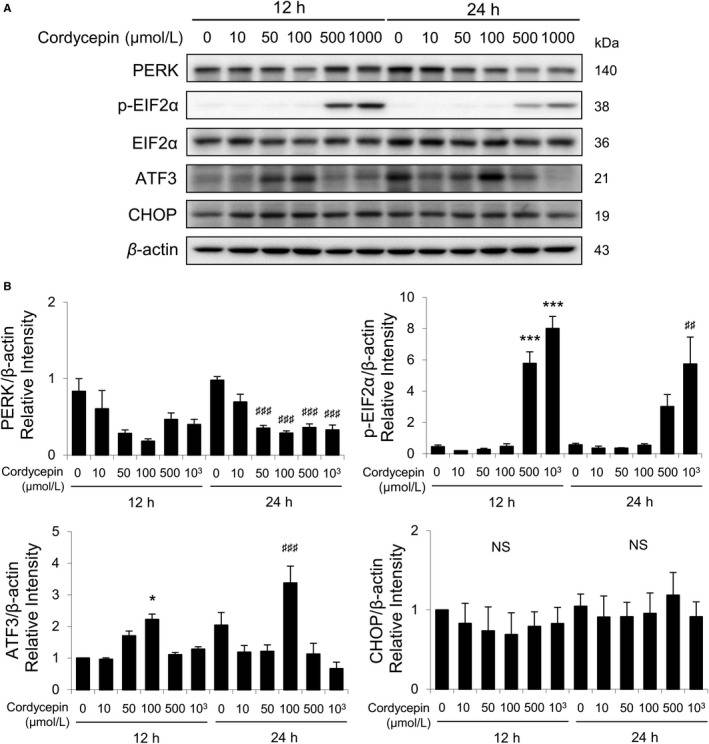 Fig. 3 Cordycepin-induced apoptosis of MA-10 cells by activating PERK/EIF2α signaling pathways. A, Western blot analysis for the expression of total and phosphorylated EIF2α, total PERK, ATF3, and CHOP in MA-10 cells treated with 0, 10, 50, 100, 500, or 1000 μmol/L cordycepin for 12 or 24 h, respectively. B, Quantification of bar graphs shows the IOD of EIF2α, phosphor-EIF2α, PERK, ATF3, and CHOP, which were normalized with β-actin (43 kDa) in each lane, respectively. (Chang MM, et al., 2019)
Fig. 3 Cordycepin-induced apoptosis of MA-10 cells by activating PERK/EIF2α signaling pathways. A, Western blot analysis for the expression of total and phosphorylated EIF2α, total PERK, ATF3, and CHOP in MA-10 cells treated with 0, 10, 50, 100, 500, or 1000 μmol/L cordycepin for 12 or 24 h, respectively. B, Quantification of bar graphs shows the IOD of EIF2α, phosphor-EIF2α, PERK, ATF3, and CHOP, which were normalized with β-actin (43 kDa) in each lane, respectively. (Chang MM, et al., 2019)
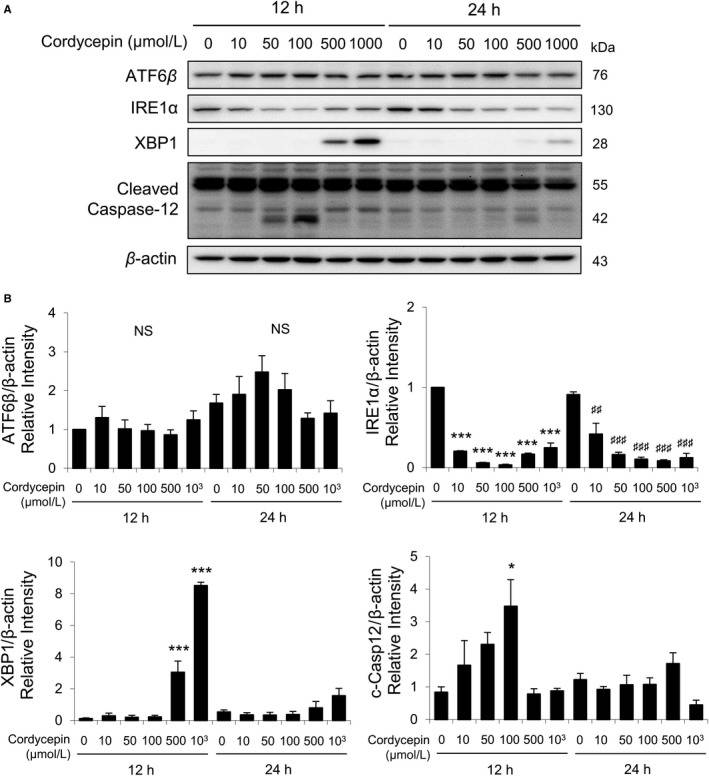 Fig. 4 Cordycepin-induced apoptosis of MA-10 cells by activating ATF6 and IRE1 signaling pathways. A, Western blot analysis for the expression of total ATF6β, IRE1α, XBP1, total, and cleaved caspase-12 in MA-10 cells treated with 0, 10, 50, 100, 500, or 1000 μmol/L cordycepin for 12 or 24 h, respectively. B, Quantification of bar graphs show that the IOD of ATF6β, IRE1α, XBP1, and cleaved caspase-12 (c-Casp12), which were normalized with β-actin (43 kDa) in each lane, respectively. (Chang MM, et al., 2019)
Fig. 4 Cordycepin-induced apoptosis of MA-10 cells by activating ATF6 and IRE1 signaling pathways. A, Western blot analysis for the expression of total ATF6β, IRE1α, XBP1, total, and cleaved caspase-12 in MA-10 cells treated with 0, 10, 50, 100, 500, or 1000 μmol/L cordycepin for 12 or 24 h, respectively. B, Quantification of bar graphs show that the IOD of ATF6β, IRE1α, XBP1, and cleaved caspase-12 (c-Casp12), which were normalized with β-actin (43 kDa) in each lane, respectively. (Chang MM, et al., 2019)
Description: Species: human, Caucasian male 22 years;
Tissue: testis;
Tumor: carcinoma, embryonal...
Description: Complex type germ cell tumor of human embryonic testis origin.
Description: Complex type germ cell tumor of human embryonic testis origin.
Description: Complex type germ cell tumor of human embryonic testis origin.
Description: A human embryonal carcinoma cell line derived from a human testicular germ cell tumour.

