Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

Skin Tumor Cells
- Product List
- Background
- Applications
- Scientific Data
As the largest organ of the body and the primary barrier against the external environment, the skin is susceptible to a variety of insults, including ultraviolet radiation, chemical exposure, and genetic predisposition, which can lead to tumor formation. Skin tumor cells originate from the epidermal layer of the skin, composed of rapidly dividing cells constantly exposed to environmental factors capable of inducing genetic mutations. These mutations can disrupt the normal regulatory mechanisms of cell growth and division, leading to the unchecked proliferation that characterizes tumor formation. The cellular origins of skin tumor cells are diverse, reflecting the heterogeneity of the epidermis. For example, melanocytes, the cells that produce the pigment melanin, can give rise to melanoma when they become cancerous. Similarly, basal cells and squamous cells, which are found in the lower and upper layers of the epidermis, respectively, can transform into basal cell carcinoma and squamous cell carcinoma.
Molecular Biology of Skin Tumor Cells
- Genetic alterations. Skin tumor cells often harbor mutations in genes that regulate cell growth, differentiation, and apoptosis. Common genetic alterations include mutations in the p53 tumor suppressor gene, the RAS/RAF/MEK/ERK pathway, and the PI3K/AKT/mTOR pathway. These mutations can lead to uncontrolled cell proliferation and resistance to cell death, contributing to tumor development and progression.
- Cellular signaling pathways. Cellular signaling pathways are critical for the communication between cells and their environment. In skin tumor cells, dysregulation of these pathways can result in altered cell behavior. The Wnt/β-catenin, Notch, and Hedgehog pathways are particularly important in the context of skin tumor development, as they play key roles in skin stem cell maintenance and differentiation.
- Tumor microenvironment. The tumor microenvironment, which includes immune cells, fibroblasts, and extracellular matrix components, significantly influences skin tumor cell behavior. Researchers study the interactions between tumor cells and their microenvironment to identify new therapeutic targets and develop treatments that modulate the tumor microenvironment to inhibit tumor growth and metastasis.
Regenerative Medicine and Tissue Engineering
Skin tumor cells are also being explored in the field of regenerative medicine. Researchers are developing three-dimensional skin models that incorporate tumor cells. These models can be used to study tumor-stroma interactions and test the effects of potential treatments in a more physiologically relevant environment. Investigating the potential of using modified skin tumor cells for therapeutic purposes, such as delivering anti-cancer agents directly to tumors, represents a novel approach to cancer therapy.
Drug Discovery and Development
Skin tumor cells serve as a vital resource in the preclinical phase of drug development. Laboratory-grown skin tumor cells are used to screen potential drug candidates for efficacy and toxicity. This process helps identify compounds that can inhibit tumor growth or promote apoptosis in cancer cells. By implanting human skin tumor cells into immunocompromised mice, researchers can create xenograft models that mimic human tumor behavior. These models are essential for testing new therapeutic agents in a biological context that closely resembles human physiology.
Genetic and Molecular Profiling
Advancements in genetic profiling technologies, such as next-generation sequencing (NGS), have made it possible to analyze the genomic landscape of skin tumor cells extensively. Identifying specific genetic alterations can lead to the discovery of biomarkers that predict treatment response or disease progression. This information can guide clinical decision-making and improve personalized treatment strategies. By studying the various subclones within a tumor, researchers can gain insights into how different cell populations respond to therapies, aiding in the development of more effective treatment regimens.
Effect of 13-Butoxyberberine Bromide on Skin Cancer A431 Cell Migration and Invasion
Cancer metastasis is the leading cause of cancer-related death. Most cancer deaths are caused by cancer metastasis, not the primary tumor. Metastasis is a multistep process that includes cell invasion, cancer cells entering blood vessels, leaving the circulatory system, and colonizing distant organs. It is currently known that berberine has many pharmacological activities including antioxidant, anti-inflammation, antibacterial, anticholinergic, antihypertensive, and anticancer activities.
The effect of 13-butoxy berberine bromide on A431 cell migration was examined using a transwell migration assay. The results suggested that 13-butoxy berberine bromide significantly suppressed cell migration in A431 cells compared to the control group (0.5% DMSO) (Fig. 1A). Cell migration in A431 cells was suppressed by approximately 40.4%, 56.6%, and 75.4% after treatment with 1, 3, and 5 µM of 13-butoxy berberine bromide, respectively (Fig. 1B). These results suggested that 13-butoxy berberine bromide could inhibit cell migration in A431 cells.
The effect of 13-butoxy berberine bromide on cell invasion in A431 cells was determined using a transwell invasion assay. The results indicated that 13-butoxy berberine bromide suppressed A431 cell invasion across the Matrigel-coated membrane filter compared to the control group (0.5% DMSO) (Fig. 2A). Cell invasion in A431 cells was reduced by 31.3%, 45.2%, and 71.5% after treatment with 1, 3, and 5 µM of 13-butoxy berberine bromide, respectively (Fig. 2B). These results suggested that 13-butoxy berberine bromide significantly inhibited A431 cell invasion.
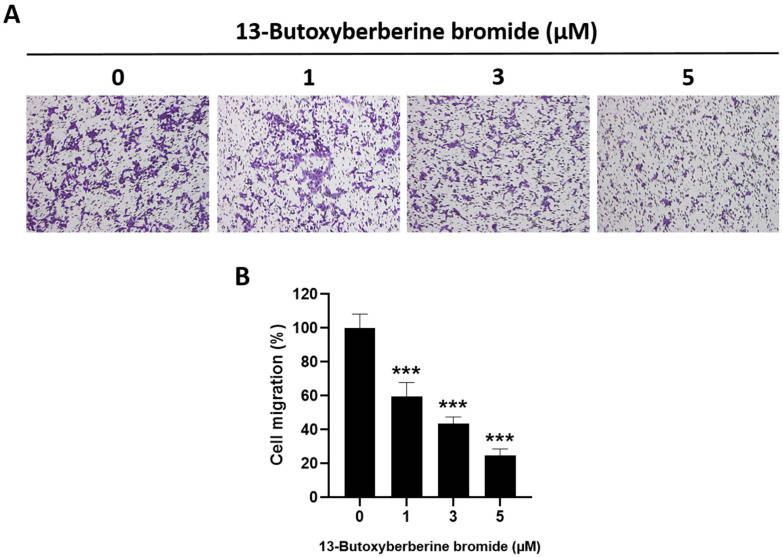 Fig. 1 Inhibitory effect of 13-butoxy berberine bromide on cell migration in A431 cells. (A) Morphology of migrated A431 cells after staining with crystal violet under an inverted microscope. (B) The histogram shows the percentage of cell migration after incubation with 13-butoxy berberine bromide. (Laomethakorn P, et al, 2023)
Fig. 1 Inhibitory effect of 13-butoxy berberine bromide on cell migration in A431 cells. (A) Morphology of migrated A431 cells after staining with crystal violet under an inverted microscope. (B) The histogram shows the percentage of cell migration after incubation with 13-butoxy berberine bromide. (Laomethakorn P, et al, 2023)
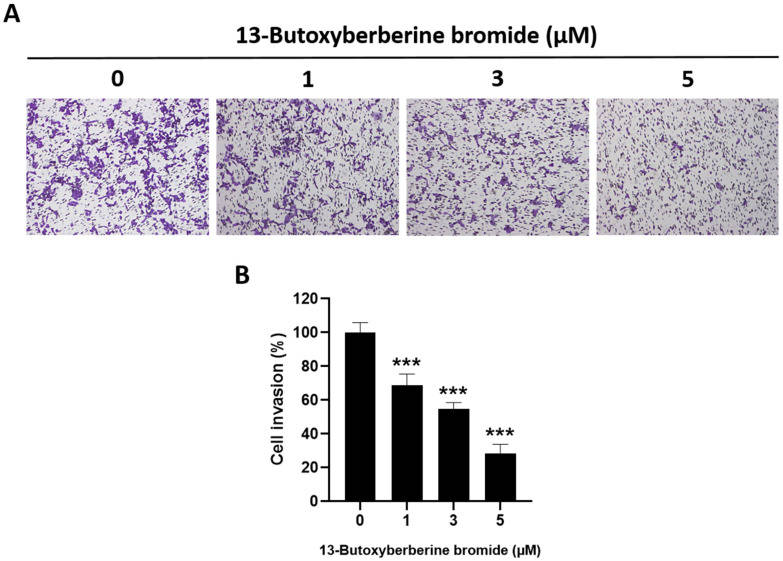 Fig. 2 Inhibitory effect of 13-butoxy berberine bromide on cell invasion in A431 cells. (A) Morphology of invaded A431 cells after staining with crystal violet under an inverted microscope. (B) The histogram shows the percentage of cell invasion after incubation with 13-butoxyberberine bromide. (Laomethakorn P, et al, 2023)
Fig. 2 Inhibitory effect of 13-butoxy berberine bromide on cell invasion in A431 cells. (A) Morphology of invaded A431 cells after staining with crystal violet under an inverted microscope. (B) The histogram shows the percentage of cell invasion after incubation with 13-butoxyberberine bromide. (Laomethakorn P, et al, 2023)
Secretome Analysis of HaCaT and A431 Cells
Cutaneous squamous cell carcinoma (cSCC) is the most prominent tumor of non-melanoma skin cancers and the most aggressive tumor among keratinocyte carcinoma of the skin, showing a high potential for local invasion and metastasis. The secretome of human A431 cancer cells and HaCaT keratinocytes were analyzed and compared by mass spectrometry.
Hierarchical clustering of the label-free quantification (LFQ) values separates CM-proteins of HaCaT cells from those of A431 cells (Fig. 3A). The Volcano plot analysis indicates major differences between both secretomes (Fig. 3B). In total, 1577 proteins were identified of which 299 were more abundant in the conditioned medium (CM) of HaCaT cells and 383 more abundant in the CM of A431 cells. For a more detailed analysis of the secreted proteins in the CM, the differentially abundant proteins were functionally clustered using Cytoscape and the Clue-GO plug-in in combination with the Reactome-Pathway database. Proteins related to the GO terms: formation of the cornified envelope, cell-cell communication, signaling by RTKs, assembly of collagen fibrils, and platelet activation, signaling, and aggregation were enriched in the HaCaT secretome. In contrast, the more abundant secreted proteins of A431 cells were involved in the GO terms: metabolism, metabolism of carbohydrates, metabolism of nucleotides, metabolism of proteins, and post-translational protein phosphorylation. This shows that many secreted proteins of A431 are involved in the cellular metabolism, in contrast to the secreted proteins of HaCaT cells. Proteins of the following pathways were enriched in the CM of both cell lines: cell cycle, extracellular matrix organization, innate immune system, non-integrin membrane-ECM interactions, signaling by WNT, signaling by NOTCH, neutrophil degranulation, cellular responses to stress, and axon guidance.
Many ECM proteins were more abundant in HaCaT cells, including the proteins serpin B7 (SERPINB7), matrix metalloproteinase 13 and 2 (MMP13, MMP2), gelsolin (GSN), fibronectin (FN1), matrix-remodeling-associated protein 5 (MXRA5), and sparc (SPARC) (Fig. 3C). Additionally, desmoglein-4 (DSG4) and desmocollin-3 (DSC3) are significantly increased in the CM of HaCaT cells (Fig. 3C). Both proteins are components of intercellular desmosome junction and are expressed in particular in hair follicles. In the A431-CM significantly increased levels of plasma alpha-L-fucosidase 2 (FUCA2), leucine-rich alpha-2-glycoprotein-1 (LRG1), melanoma-associated antigen 4 (MAGEA4), AXL receptor tyrosine kinase (AXL), and the receptor tyrosine kinase (RTK) epidermal growth factor receptor (EGFR) were identified (Fig. 3C). All these proteins are well-known proto-oncoproteins and up-regulated in different types of cancer. Furthermore, vascular endothelial growth factor A (VEGFA), an important protein for tumor vascularization, was exclusively identified in the A431-CM (Fig. 3B, D). Several members of the disintegrin and metalloproteinase domain-containing protein (ADAM) family were found to be more abundant in A431-CM (Fig. 3B, D). ADAM proteins are important sheddases of ERBB ligands, AXL, and LRG1, which are significantly increased in the CM of A431 cells. An apoptosis assay revealed that the FCS starvation process does not affect the apoptosis rate either in HaCaT (Fig. 3E) or in A431 (Fig. 3F) cells.
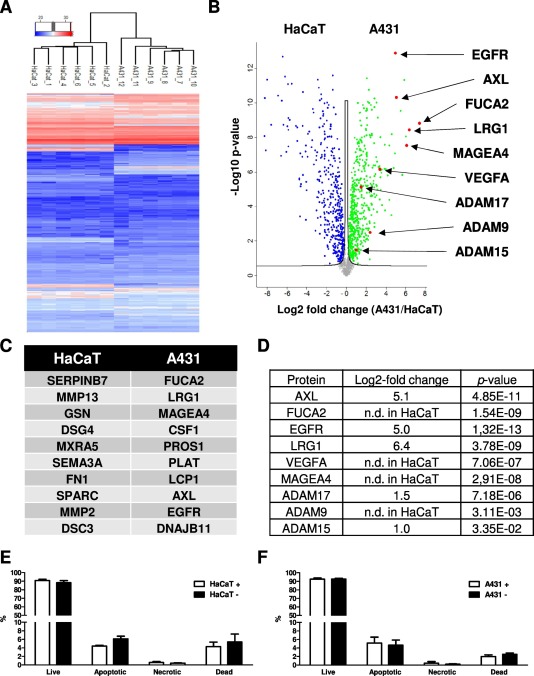 Fig. 3 (A) Unsupervised hierarchical clustering of normalized LFQ intensity values. The secretomes of HaCaT and A431 cells are separated. (B) Volcano plot analysis of log2 fold changes (A431/HaCaT-CM). (C) List of the top 10 proteins showing the highest differences in abundance in HaCaT or A431 respectively. (D) Table showing the Log2-fold change and P-value of the prominent proteins significantly increased in the A431-CM. (Hoesl C, et al., 2020)
Fig. 3 (A) Unsupervised hierarchical clustering of normalized LFQ intensity values. The secretomes of HaCaT and A431 cells are separated. (B) Volcano plot analysis of log2 fold changes (A431/HaCaT-CM). (C) List of the top 10 proteins showing the highest differences in abundance in HaCaT or A431 respectively. (D) Table showing the Log2-fold change and P-value of the prominent proteins significantly increased in the A431-CM. (Hoesl C, et al., 2020)
Description: Species: human male 65 year old;
Tissue: skin;
Tumor: melanoma;
Derived from: a lymph node metastasis
Description: Species: human, Caucasian male;
Tissue: metastatic cutaneous nodule;
Tumor: melanoma
Description: Species: human male;
Tissue: subcutaneous metastasis;
Tumor: malignant melanoma
Description: Species: human;
Tissue: skin;
Tumor: melanoma
Description: Derived from a 14-18 week old human foetus. The cells support growth of CMV and HSV...
Description: Malignant trichilemmal cyst cells. Strongly laminin positive on cell surface. Serum-free culturable.
Description: A cell line derived from human skin squamous cell carcinoma having anaplastic epithelioid features.
Description: This is a human cell line with the normal 46,XY karyotype. The modal chromosome number...
Description: Microvascular endothelial cells isolated from human foreskins were transfected with...
Description: The cells have a finite lifespan of about 25 serial passages from the tissue of origin...
Description: Species: human - male, 60 years old, Caucasian
Tumorigenecity: Yes, in nude mice;...

