Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
HMEC-1
Cat.No.: CSC-C9191W
Species: Human
Source: dermal endothelium
Morphology: endothelial-like
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Ades created the HMEC-1 cell line in 1992 by using the SV40 large T antigen gene to transfect human dermal microvascular endothelial cells to achieve immortality. The HMEC-1 cell line maintains primary endothelial cell characteristics and functions as well as standard markers CD31, PECAM-1 (VWF), and VE-Cadherin. HMEC-1 cells show a "cobblestone" morphology while they attach to surfaces and develop structures similar to capillaries during cell culture. Under serum deprivation these cells display consistent morphology and demonstrate significant proliferation density reaching three to seven times the density of other endothelial cells. The unique properties and stability of HMEC-1 cells allow them to be essential for researching angiogenesis and endothelial barrier function and serve as models for understanding vascular diseases such as cancer and diabetic retinopathy while testing drug impacts on vascular permeability and inflammation. Artificial vascular and tissue repair models rely on them as foundational materials.
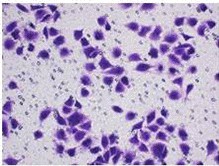 Fig. 1. HMEC-1 cells (Yang Y, Wang J, et al., 2021).
Fig. 1. HMEC-1 cells (Yang Y, Wang J, et al., 2021).
Moderate Levels of Haem can Relieve the Inhibition of Hyperoxia on HMEC-1 Proliferation
ROP is prevalent in premature infants due to enhanced perinatal care and neonatology, mainly caused by retinal angiogenesis stunted by relative hyperoxia, suppressing VEGF expression. While BACH1 inhibits VEGF, haem degrades BACH1, but its effect on endothelial cells in ROP is unknown.
Lan's team model phase I ROP's hyperoxia using HMEC-1 cells exposed to 40% hyperoxia. Various haem doses are tested on cell proliferation, migration, and angiogenesis. Under hyperoxic conditions, HMEC-1 cell proliferation is inhibited, but 5–40 μM haem enhances it, while 80 μM or more inhibits it. Under normoxic conditions, 10–40 μM haem boosts proliferation, whereas 160 μM or more inhibits it. The optimal dose for HMEC-1 proliferation under both conditions is 20 μM haem (Fig. 1a). Over time, proliferation of HMEC-1 peaks at 72 hours. The proliferation rate with 20 μM haem is higher than the control under both conditions, although no significant difference exists between the 20 μM haem hyperoxia and normoxia control groups, indicating that 20 μM haem alleviates hyperoxia-induced inhibition (Fig. 1b). The EdU assay shows green fluorescence in proliferating cells (Fig. 1c). Analysis reveals that under hyperoxic conditions, total cell number, proliferative cell number, and percentage decrease. Treatment with 20 μM haem increases these metrics under both hyperoxic and normoxic conditions. No significant difference is found between the 20 μM haem hyperoxia group and the normoxic control, confirming that 20 μM haem alleviates hyperoxia-induced proliferation inhibition (Fig. 1d-f).
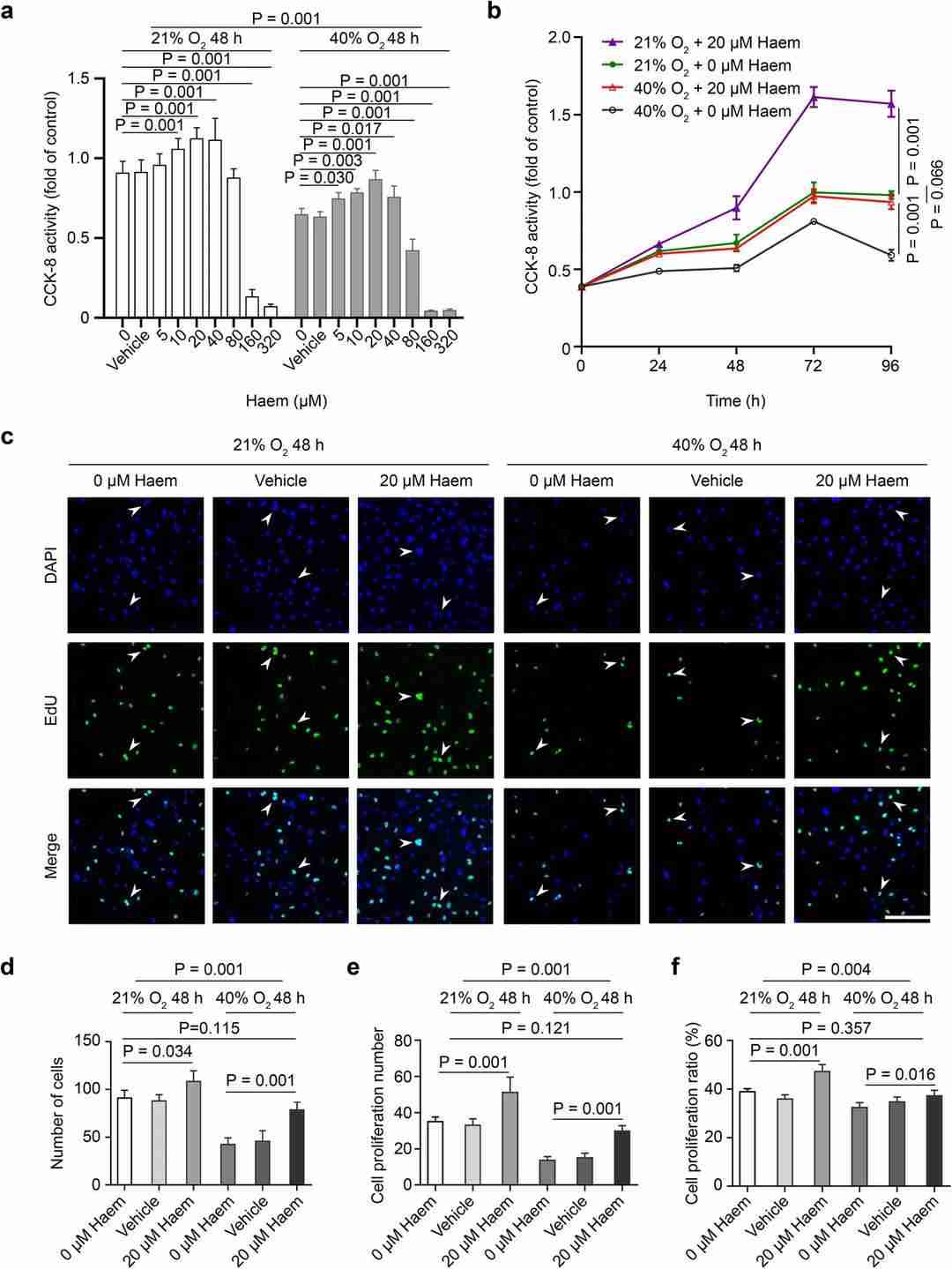 Fig. 1. Effect of haem on HMEC-1 cell proliferation (Jian L, Mei Y, et al., 2021).
Fig. 1. Effect of haem on HMEC-1 cell proliferation (Jian L, Mei Y, et al., 2021).
Doxorubicin Induces Senescence in HMEC-1 Cells
Aging impacts chronic disease development, affecting endothelial and platelet functions and involving cellular senescence. Senescent cells secrete bioactive molecules through SASP, potentially influencing platelet behavior. Venturini et al. investigated how SASP factors from senescent endothelial cells, induced by doxorubicin, affect platelet activity.
To generate senescent endothelial cells, HMEC-1 cells were treated with doxorubicin at concentrations of 0.05, 0.1, and 0.5 µM for 48 or 96 hours. Proliferation decreased at all three concentrations and further declined with longer exposure (Fig. 2A). SA-β-Gal assays confirmed increased senescence with higher doxorubicin doses (Fig. 2B-D). Even the lowest dose (0.05 µM) induced senescence, with higher doses compromising cell viability, especially at 96 hours (Fig. 2B). Thus, 0.05 µM doxorubicin was used for senescence induction in subsequent experiments. Senescent markers increased with time, peaking after 72 hours (Fig. 2D). CDKN1A mRNA levels also rose after 72 hours, supporting senescence induction (Fig. 2E).
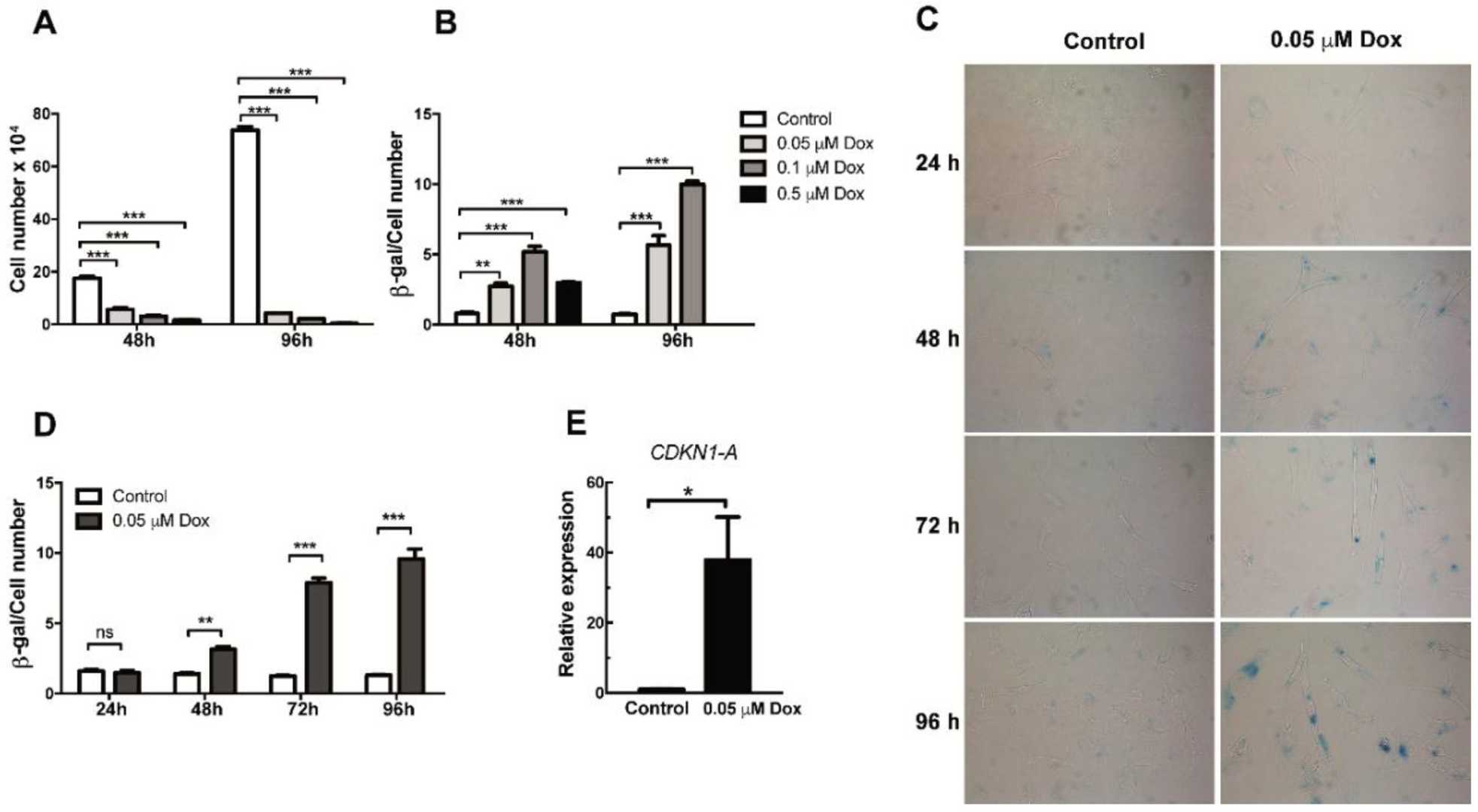 Fig. 2. Analysis of proliferation and senescence in doxorubicin-treated HMEC-1 cells (Venturini W, Olate-Briones A, et al., 2020).
Fig. 2. Analysis of proliferation and senescence in doxorubicin-treated HMEC-1 cells (Venturini W, Olate-Briones A, et al., 2020).
Monolayer cells can be propagated through passaging, where cells are detached from the solid surface using enzymes or mechanical methods, and then seeded into new culture vessels.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Helpful
The customer support team in Creative Bioarray was helpful and accommodating, making the entire purchasing process a pleasure.
03 Mar 2023
Ease of use
After sales services
Value for money
Write your own review