- You are here: Home
- Services
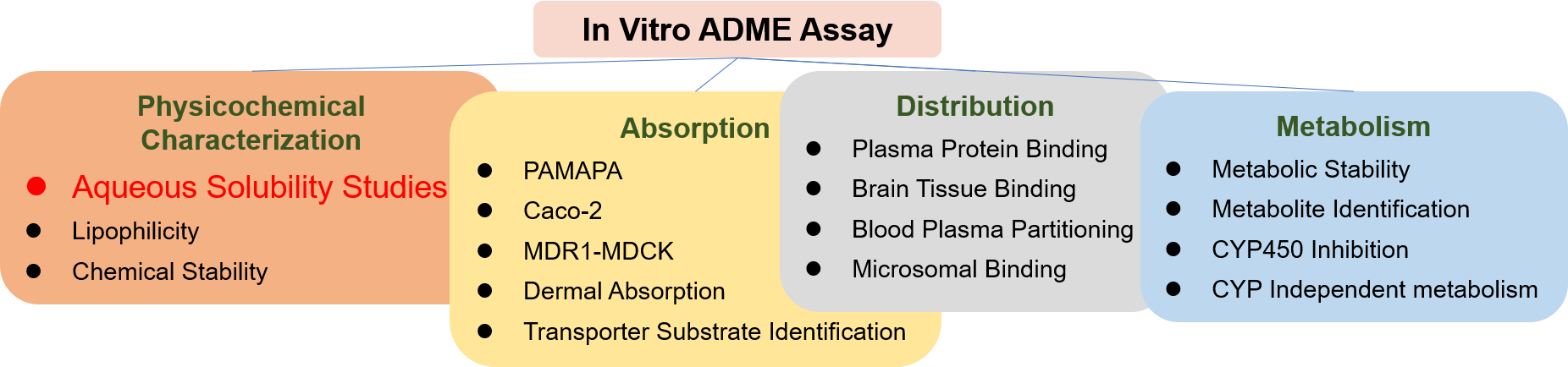
- In Vitro DMPK Services
- Physicochemical Characterization Assays
- Aqueous Solubility Assays
Services
-
Cell Services
- Cell Line Authentication
- Cell Surface Marker Validation Service
-
Cell Line Testing and Assays
- Toxicology Assay
- Drug-Resistant Cell Models
- Cell Viability Assays
- Cell Proliferation Assays
- Cell Migration Assays
- Soft Agar Colony Formation Assay Service
- SRB Assay
- Cell Apoptosis Assays
- Cell Cycle Assays
- Cell Angiogenesis Assays
- DNA/RNA Extraction
- Custom Cell & Tissue Lysate Service
- Cellular Phosphorylation Assays
- Stability Testing
- Sterility Testing
- Endotoxin Detection and Removal
- Phagocytosis Assays
- Cell-Based Screening and Profiling Services
- 3D-Based Services
- Custom Cell Services
- Cell-based LNP Evaluation
-
Stem Cell Research
- iPSC Generation
- iPSC Characterization
-
iPSC Differentiation
- Neural Stem Cells Differentiation Service from iPSC
- Astrocyte Differentiation Service from iPSC
- Retinal Pigment Epithelium (RPE) Differentiation Service from iPSC
- Cardiomyocyte Differentiation Service from iPSC
- T Cell, NK Cell Differentiation Service from iPSC
- Hepatocyte Differentiation Service from iPSC
- Beta Cell Differentiation Service from iPSC
- Brain Organoid Differentiation Service from iPSC
- Cardiac Organoid Differentiation Service from iPSC
- Kidney Organoid Differentiation Service from iPSC
- GABAnergic Neuron Differentiation Service from iPSC
- Undifferentiated iPSC Detection
- iPSC Gene Editing
- iPSC Expanding Service
- MSC Services
- Stem Cell Assay Development and Screening
- Cell Immortalization
-
ISH/FISH Services
- In Situ Hybridization (ISH) & RNAscope Service
- Fluorescent In Situ Hybridization
- FISH Probe Design, Synthesis and Testing Service
-
FISH Applications
- Multicolor FISH (M-FISH) Analysis
- Chromosome Analysis of ES and iPS Cells
- RNA FISH in Plant Service
- Mouse Model and PDX Analysis (FISH)
- Cell Transplantation Analysis (FISH)
- In Situ Detection of CAR-T Cells & Oncolytic Viruses
- CAR-T/CAR-NK Target Assessment Service (ISH)
- ImmunoFISH Analysis (FISH+IHC)
- Splice Variant Analysis (FISH)
- Telomere Length Analysis (Q-FISH)
- Telomere Length Analysis (qPCR assay)
- FISH Analysis of Microorganisms
- Neoplasms FISH Analysis
- CARD-FISH for Environmental Microorganisms (FISH)
- FISH Quality Control Services
- QuantiGene Plex Assay
- Circulating Tumor Cell (CTC) FISH
- mtRNA Analysis (FISH)
- In Situ Detection of Chemokines/Cytokines
- In Situ Detection of Virus
- Transgene Mapping (FISH)
- Transgene Mapping (Locus Amplification & Sequencing)
- Stable Cell Line Genetic Stability Testing
- Genetic Stability Testing (Locus Amplification & Sequencing + ddPCR)
- Clonality Analysis Service (FISH)
- Karyotyping (G-banded) Service
- Animal Chromosome Analysis (G-banded) Service
- I-FISH Service
- AAV Biodistribution Analysis (RNA ISH)
- Molecular Karyotyping (aCGH)
- Droplet Digital PCR (ddPCR) Service
- Digital ISH Image Quantification and Statistical Analysis
- SCE (Sister Chromatid Exchange) Analysis
- Biosample Services
- Histology Services
- Exosome Research Services
- In Vitro DMPK Services
-
In Vivo DMPK Services
- Pharmacokinetic and Toxicokinetic
- PK/PD Biomarker Analysis
- Bioavailability and Bioequivalence
- Bioanalytical Package
- Metabolite Profiling and Identification
- In Vivo Toxicity Study
- Mass Balance, Excretion and Expired Air Collection
- Administration Routes and Biofluid Sampling
- Quantitative Tissue Distribution
- Target Tissue Exposure
- In Vivo Blood-Brain-Barrier Assay
- Drug Toxicity Services
Aqueous Solubility Assays
Creative Bioarray provides Aqueous Solubility Assays to help customers accurately evaluate the drug solubility, allowing customers to make early modifications to their drugs to alleviate difficulties in the drug research process and improve their bioavailability.
Why is it necessary to determine drug solubility?
- Low solubility can impair the formation of in vitro DMPK or biological data quality, which can be a significant stumbling block in the drug discovery and development process.
- Low solubility may lead to problems in generating suitable formulations for in vivo pharmacokinetic studies.
- Unknown solubility may lead to problems in the absorption of the drug after oral administration. If the drug solubility is known, then the correct and reliable compound concentration will be available.
Brief Protocol
Thermodynamic and kinetic solubility assays are two commonly used laboratory methods to determine drug solubility.
- Kinetic solubility assay (The most common method)
Advantages: Kinetic solubility assay is often used in the early drug discovery process as it is fast and has higher throughput. In this case, the compound does not need to dissolve by overcoming any crystal forces in the solid state because these have been overcome before the experiment by DMSO dissolution.
Materials: Compounds already fully dissolved in an organic solvent.
- After first dissolving the solid compounds in DMSO, each compound's linear series of dilutions is added to an aqueous buffer, and precipitate formation is observed while the compounds are incompletely dissolved.
- After filtration or spin-down to remove the insoluble, solubility can be measured using HPLC-UV or LC-MS/MS.
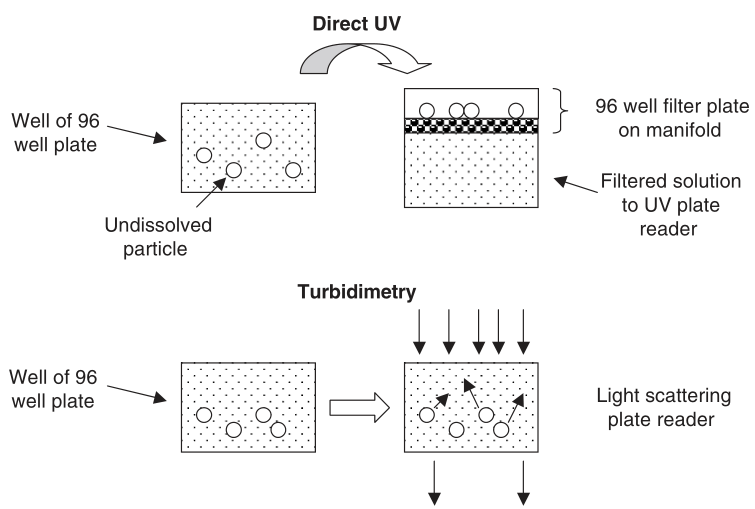 Figure 2. Two common approaches for kinetic solubility assays (Edward H, 2005).
Figure 2. Two common approaches for kinetic solubility assays (Edward H, 2005).
- Thermodynamic solubility assay
Advantages: Thermodynamic solubility assay is typically a late-discovery screening detection that calculates the equilibrium solubility of a test compound by measuring its concentration in a solution containing excess compound that has been allowed to reach thermodynamic equilibrium. It is useful when preparing a data package for a proposed development candidate.
Materials: Solid crystalline material in aqueous solvent as a saturated solution in equilibrium.
- Add aqueous dissolution to the excess solid compound for a long incubation period to achieve equilibrium dissolution.
- After filtration or spin-down to remove the insoluble, solubility can be measured using HPLC-UV or LC-MS/MS.
Applications
- Solubility data can be used to assess the effect of functional groups on the solubility of compounds and to guide the development of strategies.
- Solubility data can be used to interpret the results of in vitro experiments.
- Solubility can be combined with other in vitro parameters to forecast the oral pharmacokinetics or intestinal absorption of compounds.
Quotation and ordering
If you have any special needs or questions regarding our services, please feel free to contact us. We look forward to cooperating with you in the future.
References
- Kerns, Edward H., and Li Di. "Automation in pharmaceutical profiling." JALA: Journal of the Association for Laboratory Automation 10.2 (2005): 114-123.
- Kerns, Edward H., et al. "In vitro solubility assays in drug discovery." Current drug metabolism 9.9 (2008): 879-885.
Explore Other Options
For research use only. Not for any other purpose.

