Stable Cell Line Genetic Stability Testing
- Service Details
- Features
- Case Studies
- FAQ
- Explore Other Options
In biological manufacturing, cell lines' genetic stability is critical to product quality and safety. Genetic stability testing is mainly used to monitor whether genomic changes such as gene deletions, gene rearrangements, point mutations, etc. occur in cell lines during the culture process. These genomic changes can lead to changes in cell phenotype and gene expression, which can affect the quality and stability of the proteins or other biopharmaceuticals produced. Genetic stability testing is also part of regulatory requirements. It helps to improve the manufacturing process's reliability and the product's consistency and ensures that the biological product produced meets quality standards.
Creative Bioarray offers cell line stability testing services to companies and research institutes in pharmaceuticals, tissue engineering, antibody or vaccine production. Our range of advanced technologies allows testing to be performed throughout the life cycle of the producing cell line and to assess the genetic stability of the Master Cell Bank (MCB), Working Cell Bank (WCB), and End-of-Production Cells (EOPC).
Our Solutions for Cell Line Genetic Stability Testing
Supporting Advanced FISH Techniques
Fluorescence in situ hybridization (FISH) is a powerful technique that allows us to visualize the location and number of specific gene sequences within a cell's chromosomes. Creative Bioarray employs this method to assist clients in detecting chromosome rearrangements, aneuploidies, and other genetic alterations that may occur during cell line propagation. We help clients design and synthesize FISH probes, prepare chromosome spread, perform FISH on chromosome spreads, imaging, and data analysis.
Extensive Experience for MCB, WCB, and EOPC
Our team of seasoned experts has accumulated extensive experience in working with a wide variety of MCBs, WCBs, and EOPCs. This breadth of knowledge allows us to provide accurate and reliable assessments of genetic stability, regardless of the cell line or application.
Regulatory Compliance
We are committed to ensuring that our cell line genetic stability testing services comply with relevant regulatory guidelines and standards. We work closely with our clients to understand their specific regulatory requirements and tailor our processes accordingly, ensuring that the data and documentation we provide meet the necessary criteria for regulatory approval and product commercialization.
Why Choose FISH for Cell Line Genetic Stability Testing?
At Creative Bioarray, we have conducted extensive research and comparative analyses to understand the strengths and limitations of both FISH and PCR in the context of cell line genetic stability testing.
| Techniques | FISH | PCR |
| Principles | Hybridization of fluorescently labeled DNA probes to specific chromosomal regions or genetic sequences. | Exponential amplification of target DNA sequences. Detection and quantification of specific genetic changes. |
| Applications in cell line genetic stability testing |
|
|
| Advantages |
|
|
| Disadvantages |
|
|
Creative Bioarray's Stable Cell Line Genetic Stability Testing Has the Following Features
- Able to detect multiple target sequences of low copy number or multiple unknown DNA insertion sites (in hundreds of cells if you want)
- High accuracy and sensitivity
- Fast turnaround time
- Competitive pricing
Case Study
523 cells were used to detect the translocation signals of Gene X, Gene Y, and Gene Z. Fusion signals were found at 5.7% of total cells. In 50 metaphase photos, no fusion signals were found in the cell sample.
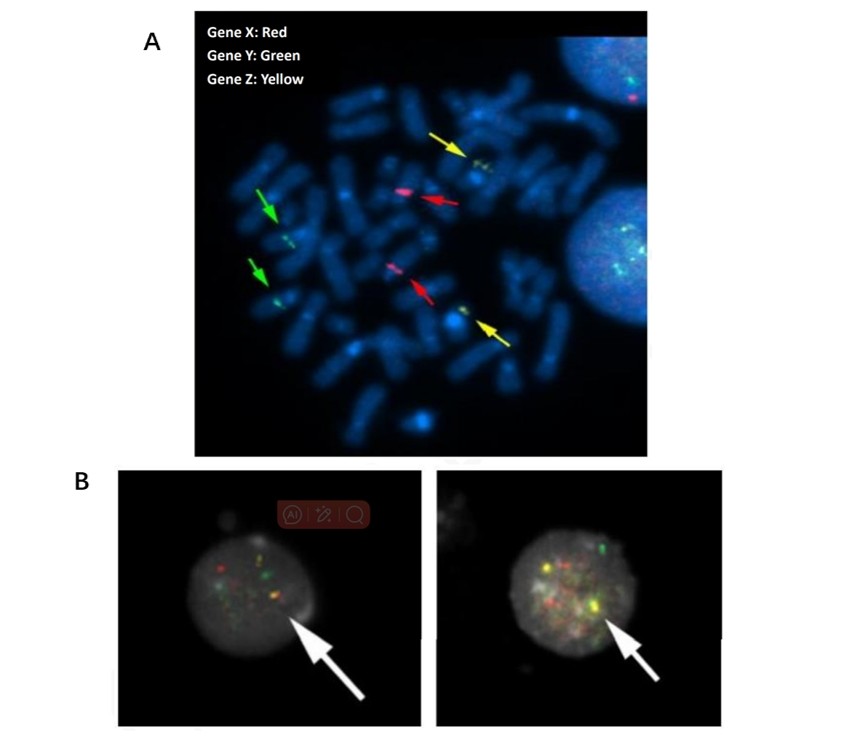 Fig. 1 (A) FISH signals on metaphase cells; (B) FISH signals on interphase nuclei.
Fig. 1 (A) FISH signals on metaphase cells; (B) FISH signals on interphase nuclei.
FAQ
1. Why is genetic stability important?
Transgenes, expressed in recombinant production cells, are subjected to mutations that may impact the product quality and consistency of the process; therefore, it is a regulatory requirement to demonstrate the stability of the expression construct over the life span of the production cells.
2. Where can I find regulatory guidelines on genetic stability?
(a). ICH Topic Q 5 B, Quality of Biotechnological Products: Analysis of the Expression Construct in Cell Lines Used for Production of rDNA Derived Protein Products, European Medicines Agency, 1996. (b). Supplement to the Points to Consider in the Production and Testing of New Drugs and Biologics Produced by Recombinant DNA Technology: Nucleic Acid Characterization and Genetic Stability, Food and Drug Administration, Center for Biologics Evaluation and Research Food and Drug Administration, 1992.
3. How does FISH contribute to validating a stable cell line?
FISH contributes to the validation of a stable cell line by providing a visual confirmation of the presence and integrity of the introduced genetic material. It ensures that the genetic modifications remain consistent and stable through successive cell generations, which is crucial for reproducible experimental results and therapeutic applications.
4. What are the advantages of using FISH over other genetic stability testing methods?
The advantages of using FISH over other genetic stability testing methods include high specificity and sensitivity in detecting specific DNA sequences, the ability to visualize and localize genetic changes directly on chromosomes, rapid and relatively straightforward procedure compared to other techniques like karyotyping or next-generation sequencing, the capability to detect both large-scale chromosomal abnormalities and small genetic changes that might be missed by other methods.
Quotation and Ordering
By choosing Creative Bioarray for your cell line genetic stability testing needs, you can be confident that you are working with a team of highly skilled professionals who are dedicated to providing accurate and reliable results. If you have any special needs or questions regarding our services, please feel free to contact us or make an online inquiry.
Explore Other Options

