High Stromal Tumor Models
Creative Bioarray, a leading Contract Research Organization (CRO), has developed the High Stromal Tumor Model Platform, which is compatible with all Cell Line-Derived Xenograft (CDX) and Patient-Derived Xenograft (PDX) models within our portfolio. With this cutting-edge platform, we can evaluate the efficacy of test compounds in settings that closely mimic the human tumor microenvironment. By leveraging this model, researchers can gain deeper insights into the intricate mechanisms of cancer, fostering a comprehensive understanding of the disease.
Tumor microenvironment (TME)
The tumor microenvironment (TME) has garnered increasing attention due to its pivotal role in tumor immune suppression, metastatic spread, local resistance, and response to targeted therapies. The TME is a complex ecosystem primarily composed of tumor cells, infiltrating immune cells-such as macrophages, dendritic cells, and lymphocytes-cancer-associated stromal cells (notably cancer-associated fibroblasts, or CAFs), endothelial cells, and adipocytes, as well as the extracellular matrix (ECM) and various signaling molecules.
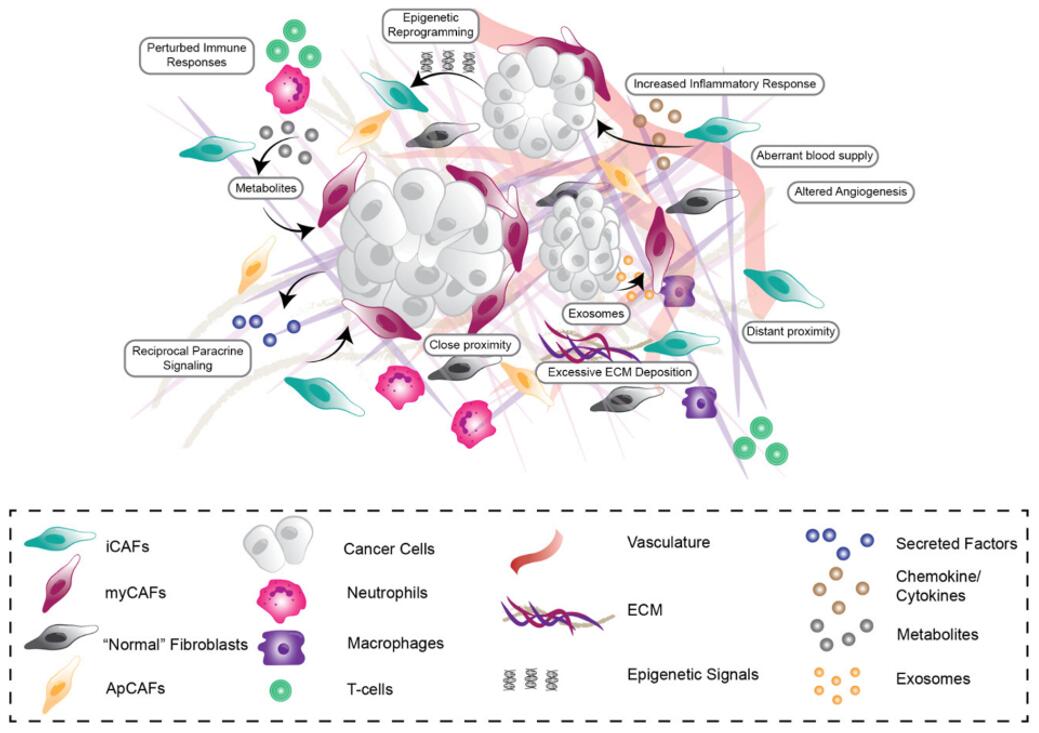 Fig. 1 Tumor microenvironment of the pancreatic tumor is highly heterogenous, consisting of cancer cells, activated cancer-associated fibroblast (CAF) subpopulations, increased deposition, remodeling and degradation of extracellular matrix (ECM), aberrant vasculature and impaired immune cell response. (Murphy et al. 2021)
Fig. 1 Tumor microenvironment of the pancreatic tumor is highly heterogenous, consisting of cancer cells, activated cancer-associated fibroblast (CAF) subpopulations, increased deposition, remodeling and degradation of extracellular matrix (ECM), aberrant vasculature and impaired immune cell response. (Murphy et al. 2021)
Cancer-associated Fibroblasts (CAFs)
CAFs, a diverse population of stromal cells characterized by their varied origins, appearances, and functions, are pivotal components of the TME. These activated cells engage in a multitude of pathways that significantly contribute to tumor progression. CAFs facilitate tumor growth, angiogenesis, invasion, and metastasis, alongside the remodeling of the ECM and the development of chemoresistance. Extensive research has underscored the indispensable role of CAFs in the intricate dance between these cells and tumor cells, influencing both the onset and advancement of cancer.
Furthermore, CAFs exert their influence on the Tumor Immune Microenvironment (TIME) by secreting an array of molecules, including cytokines, growth factors, chemokines, exosomes, and other effectors. Through these interactions, CAFs modulate the activity of tumor-infiltrating immune cells and other immune elements, sculpting an immunosuppressive TME. This environment not only suppresses immune responses but also aids cancer cells in evading the vigilant surveillance of the immune system, thereby fostering tumor survival and progression. This complex interplay between CAFs and various components of the TME highlights the critical need for targeted therapeutic strategies that can effectively modulate these interactions to combat cancer.
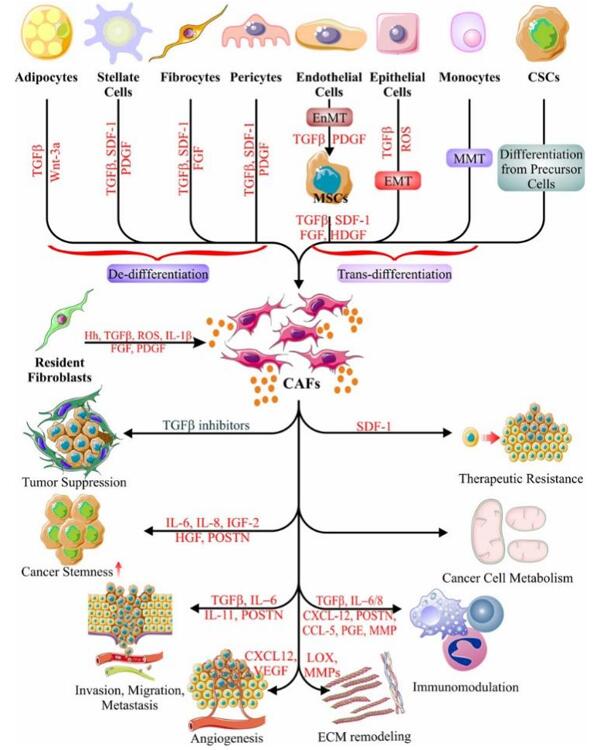 Fig. 2 Origin and function of CAFs. (Sarkar et al. 2023)
Fig. 2 Origin and function of CAFs. (Sarkar et al. 2023)
Our High Stromal Tumor Models
Creative Bioarray has pioneered the development of high-stromal cell tumor transplantation models to overcome a critical shortcoming in existing mouse models. These conventional models often fall short in accurately simulating the extensive stromal proliferation and detailed tumor environments seen in human clinical settings. Through the innovative approach of co-transplanting human mesenchymal stem cells (MSCs) alongside tumor cells, our models deliver a more authentic and reliable platform for advancing cancer research. This breakthrough not only enhances the fidelity of preclinical studies but also significantly contributes to the development of more effective cancer therapies.
High Stromal Tumor Model
Triple Humanized High Stromal Tumor Model
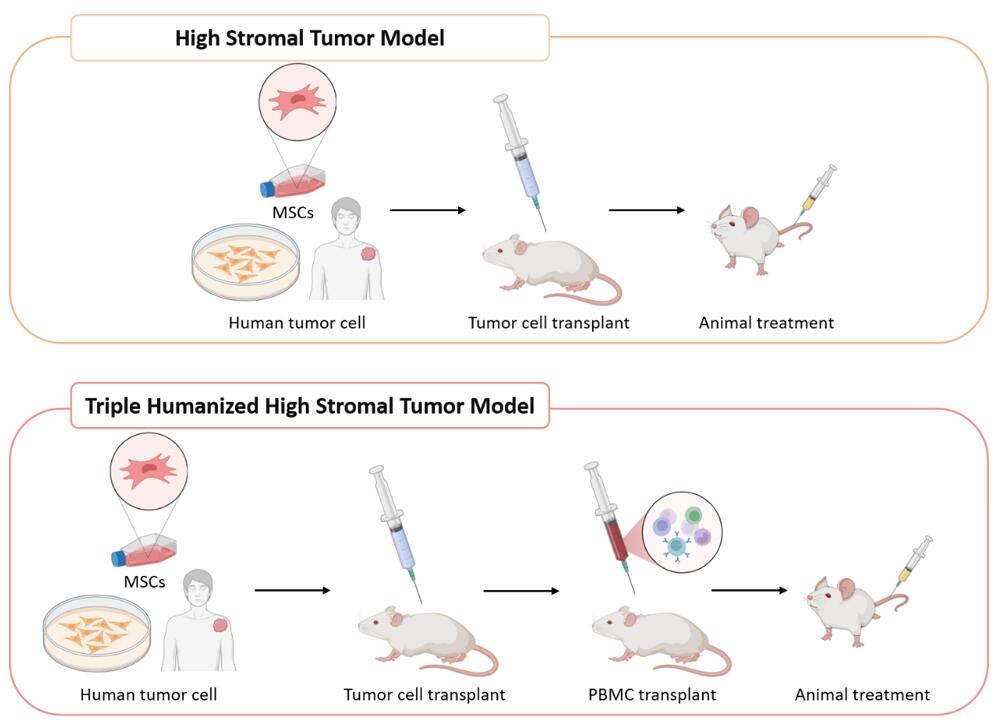 Fig. 3 Method of high stromal tumor models in Creative Bioarray.
Fig. 3 Method of high stromal tumor models in Creative Bioarray.
Key Advantages
Creative Bioarray has conducted extensive research on the tumor environments to develop the stable High Stromal Tumor Model with the following advantages:
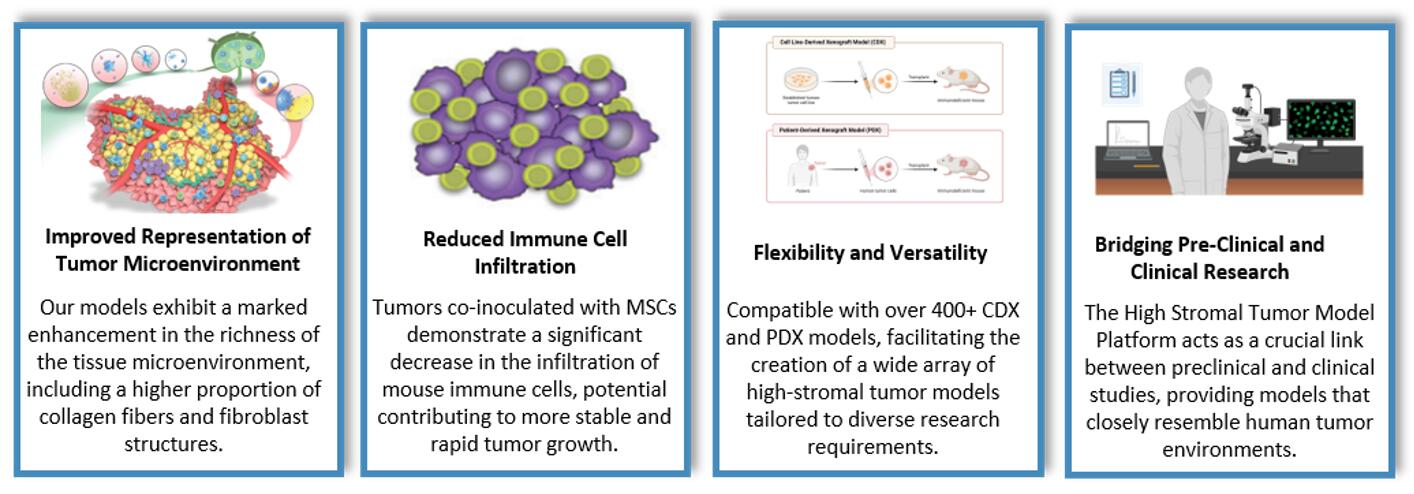 Fig. 4 Advantages of high stromal tumor model in Creative Bioarray.
Fig. 4 Advantages of high stromal tumor model in Creative Bioarray.
Example Data
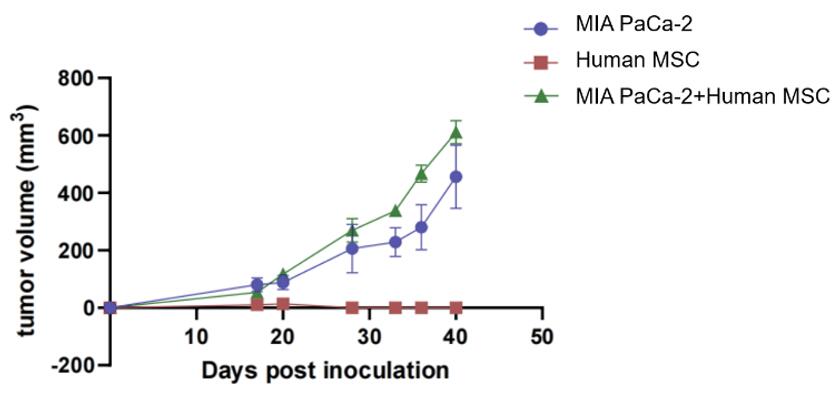 Fig. 5 Tumor volume in high stromal tumor model (MIA PaCa-2 and Human MSC inoculation). In the co-construction of the MIA PaCa-2 and MSC group, as well as the group inoculated with tumor cells alone, there was a significant increase in both the tumor growth rate and stability. Conversely, the group inoculated with MSCs alone did not produce tumors.
Fig. 5 Tumor volume in high stromal tumor model (MIA PaCa-2 and Human MSC inoculation). In the co-construction of the MIA PaCa-2 and MSC group, as well as the group inoculated with tumor cells alone, there was a significant increase in both the tumor growth rate and stability. Conversely, the group inoculated with MSCs alone did not produce tumors.
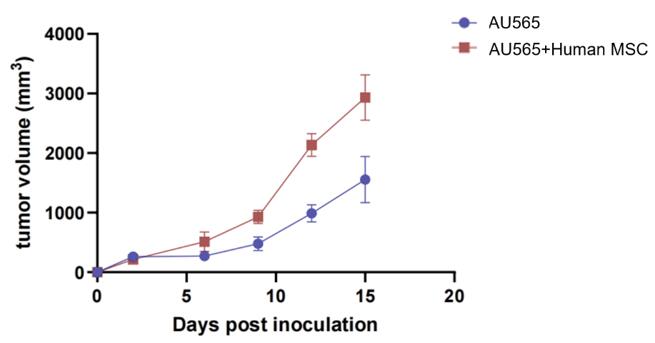 Fig. 6 Tumor volume in high stromal tumor model (AU565 and Human MSC inoculation). In the co-construction of the AU565 and MSC group, along with the group receiving only tumor cell inoculations, a notable enhancement in tumor growth rate and stability was observed.
Fig. 6 Tumor volume in high stromal tumor model (AU565 and Human MSC inoculation). In the co-construction of the AU565 and MSC group, along with the group receiving only tumor cell inoculations, a notable enhancement in tumor growth rate and stability was observed.
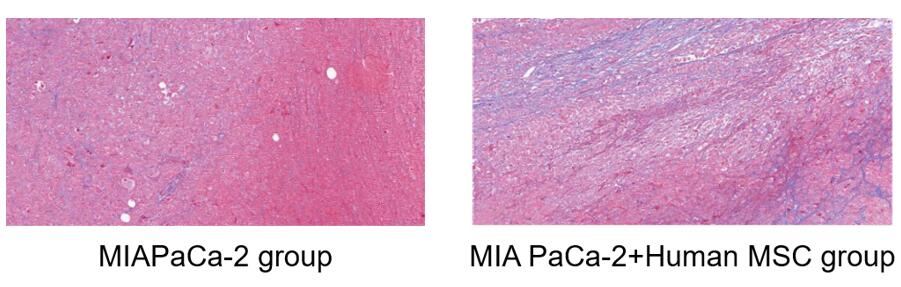 Fig.7 Masson staining of tumor tissues. The tumor co-inoculated with MSC and tumor cells showed more fibrous structures compared with tumor cells alone.
Fig.7 Masson staining of tumor tissues. The tumor co-inoculated with MSC and tumor cells showed more fibrous structures compared with tumor cells alone.
Quotation and Ordering
Creative Bioarray's mission is to be at the forefront of innovation in cancer research, offering solutions that address the most pressing challenges in the field. If you are interested in our services, please feel free to contact us anytime or submit an inquiry to us directly.
References
- Ren, X., et al. Insights gained from single-cell analysis of immune cells in the tumor microenvironment. Annual review of immunology, 2021, 39(1): 583-609.
- Murphy, K.J., et al. Dynamic stromal alterations influence tumor-stroma crosstalk to promote pancreatic cancer and treatment resistance. Cancers, 2021, 13(14): 3481.
- Sarkar, M., et al. Cancer-associated fibroblasts: the chief architect in the tumor microenvironment. Frontiers in Cell and Developmental Biology, 2023, 11: 1089068.

