In Vitro Genotoxicity
- Service Details
- Features
- FAQ
- Explore Other Options
Genotoxicity assessment serves as a critical component in evaluating the safety of various substances, including pharmaceuticals, industrial chemicals, pesticides, fungicides, food additives, cosmetic ingredients, and veterinary drugs. To thoroughly assess genotoxicity, it is essential to evaluate three primary endpoints: gene mutation, structural chromosomal aberration, and numerical chromosomal aberration, as each of these events is associated with carcinogenesis and heritable diseases.
Creative Bioarray offers a standard array of in vitro test panels, encompassing the bacterial reverse mutation test (OECD TG 471), in vitro mammalian chromosome aberration test (OECD TG 473), in vitro mammalian cell gene mutation tests (OECD TG 476 [Hprt] and TG 490 [MLA/tk]), and in vitro mammalian cell micronucleus test (OECD TG 487). Additional genotoxicity assays, such as the in vitro comet assay and genotoxicity screening, are also available.
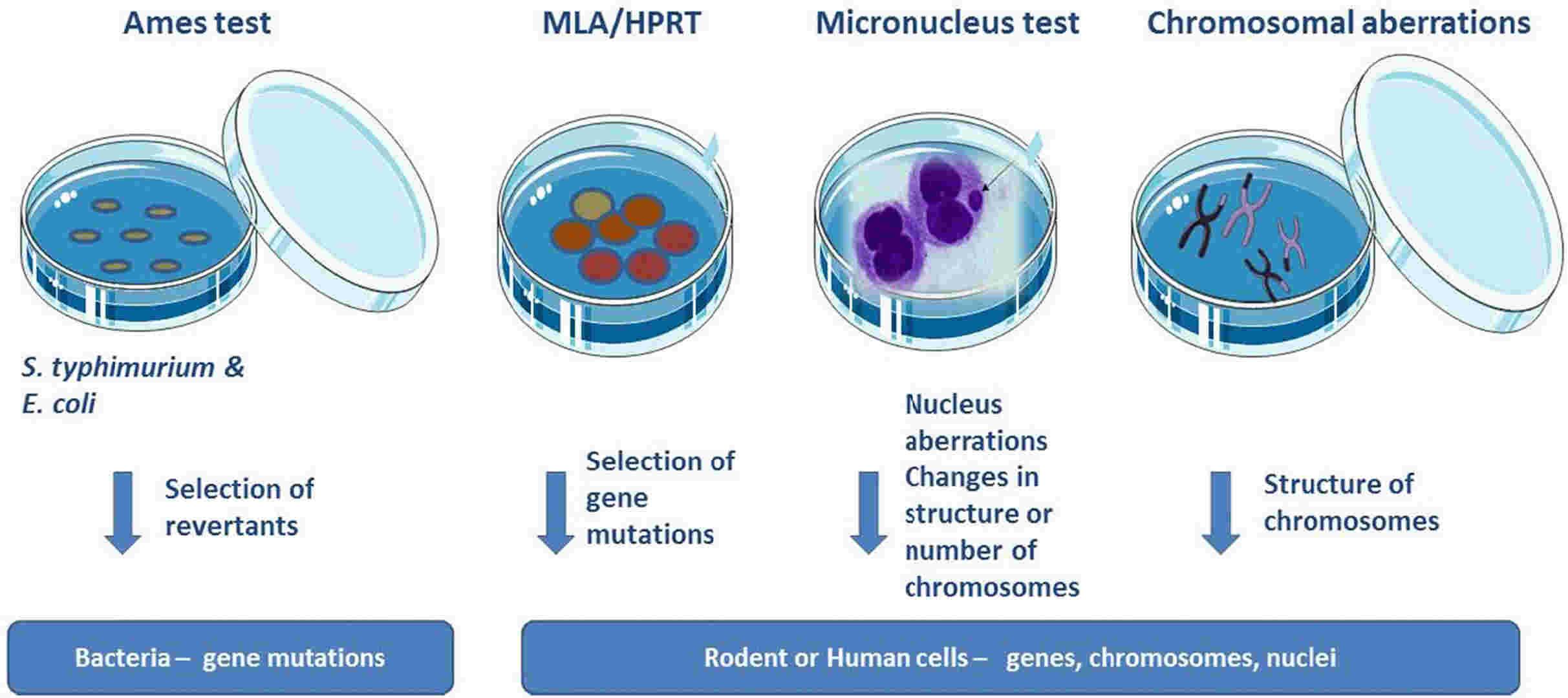 Fig. 1. In vitro test battery (Corvi R and Madia F. 2016).
Fig. 1. In vitro test battery (Corvi R and Madia F. 2016).
Why conduct in vitro genotoxicity tests?
- Genotoxicity testing is an indispensable part of drug development as these tests can identify compounds that may cause gene mutations and chromosomal damage, which are linked to cancer development.
- Early detection of potential genotoxicity of a drug or chemical before its market release helps prevent potential health risks.
- Results from these genotoxicity tests may inform adjustments in product formulation to reduce genotoxic potential and ensure compliance with relevant regulatory standards globally.
Creative Bioarray's In Vitro Cardiotoxicity Tests Include
- Bacterial reverse mutation test (Ames test) OECD 471
The Ames test utilizes histidine-dependent Salmonella typhimurium strains or tryptophan-dependent Escherichia coli strains. These strains rely on these amino acids for growth. When exposed to possible mutagens, the bacteria may undergo reverse mutation and regain the ability to synthesize histidine or tryptophan. By quantifying this restoration, the potential mutagenicity of the test substance can be evaluated. - In vitro micronucleus test mammalian cell micronucleus assay OECD 487
This test assesses the genotoxic potential of test substances by observing the formation of micronuclei in mammalian cells exposed to the substance. Micronucleus formation is typically associated with chromosomal damage, such as chromosome breaks, spindle apparatus suppression, or chromosomal aneuploidy. - In vitro mammalian chromosome aberration test OECD 473
This test detects whether structural or numerical chromosomal aberrations occur in cultured mammalian cells after exposure to a test substance. - In vitro gene mutation tests
Mouse lymphoma thymidine kinase (TK) assay OECD 490
Using L5178Y tk+/- -3.7.2C cells, this assay detects mutations at the Tk gene locus. Mutations result in a loss or reduction of TK enzyme activity, preventing DNA synthesis using analogs such as TFT, hence mutant cells cannot grow and proliferate.
In vitro mammalian cell gene mutation test (HPRT) OECD 476
Typically using Chinese hamster ovary (CHO) or lung (V79) fibroblasts, this test identifies HPRT gene mutations induced by test substances, which impair the cells' ability to metabolize analogs like 6-thioguanine (TG), preventing growth or proliferation in media containing these compounds. - In vitro comet assay
When cells are subjected to various endogenous or exogenous DNA-damaging factors, DNA strand breaks disrupt the supercoiled structure, resulting in migration during electrophoresis and forming comet-like images. Undamaged DNA remains spherical in the comet head. The DNA damage extent is assessed by observing the comet shape and migration distance. - Genotoxicity screening
Creative Bioarray offers genotoxicity screenings via Ames test, micronucleus test, and γH2AX detection.
Features
- Comprehensive: Offering a range of OECD-certified genotoxicity tests, evaluating genetic risks of chemical substances.
- High accuracy: Utilizing high-content imaging technology and machine learning models to enhance accuracy and reproducibility of tests.
- High throughput: Capable of screening multiple compounds simultaneously, suitable for early drug development phases.
- Compliance: Conducting tests in accordance with international regulatory requirements for global acceptance of results.
FAQ
1. What is genotoxicity?
Genotoxicity refers to the property of chemical substances or physical factors damaging the genetic material (DNA) of organisms. This damage can lead to gene mutations, chromosomal aberrations, or genomic instability, potentially causing diseases like cancer or hereditary diseases in reproductive cells. Genotoxic assessments are crucial components of safety testing for drugs, chemicals, and other products. In vitro and in vivo experiments can detect whether a substance adversely affects genetic material in organisms.
2. What is the AMES test, and what does it determine?
The Ames test is a widely used method employing bacteria to assess whether a chemical can induce DNA mutations in a test organism. It helps determine if a chemical poses mutagenic potential, as mutations are often associated with cancer. A positive result suggests the chemical may be mutagenic and possibly carcinogenic.
3. How is chromosomal aberration analysis conducted?
Chromosomal aberration analysis in the lab can be performed using microscopy and chromosome spreading techniques. Methods like fluorescence in situ hybridization (FISH) and G-banding are employed to distinguish and analyze chromosomal structures and changes. This analysis is vital for genetic research, cancer studies, and evaluating genotoxicity of environmental factors and drugs.
4. Does a positive test result mean the compound will definitely cause cancer?
A positive test result indicates that the compound shows genotoxic or mutagenic properties under experimental conditions. However, this does not necessarily mean it will have carcinogenic effects on humans in real-world settings. Further in vivo studies and risk assessments are typically required to fully understand its health risks.
Quotation and Ordering
Leveraging years of expertise, our team provides tailored testing strategies and comprehensive analyses to meet your unique testing needs. We also provide in vitro toxicity assays on other tissues and organs. If you have any special needs or questions regarding our services, please feel free to contact us. We look forward to working with you in the near future.
Explore Other Options

