In Vitro Cardiotoxicity
- Service Details
- Features
- FAQ
- Explore Other Options
Under the guidance of ICH S7B, conducting comprehensive and detailed cardiac toxicity assessments is crucial during new drug development. Cardiotoxicity refers to the progression of potentially harmful conditions like arrhythmia or heart failure caused by compound-induced electrophysiological derangement of the heart or muscles. It is one of the main reasons why many candidates get phased out at development or post-marketing. The recent surge in withdrawal incidents underscores the urgency of identifying and mitigating cardiac risks early in the development process.
Early cardiac safety screening offers significant advantages: first, it can help identify compounds that might pose a cardiotoxic risk early on and save valuable time and resources; second, it gives development teams key insights for optimizing molecular architecture or changing development approach, leading to higher success rates. For compounds that may cause QT interval prolongation, non-clinical hERG channel sensitivity testing is indispensable. However, standalone hERG testing has limitations, as drugs might influence multiple molecular targets, which can heighten or mitigate arrhythmia risks.
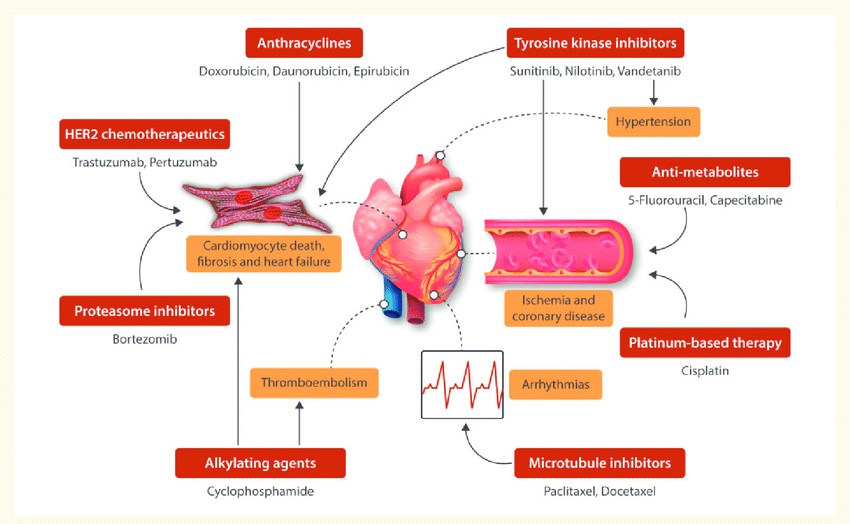 Fig. 1. Schematic of the cardiotoxic side-effects of the different chemotherapeutic agents (Sayed N, Ameen M, et al., 2019).
Fig. 1. Schematic of the cardiotoxic side-effects of the different chemotherapeutic agents (Sayed N, Ameen M, et al., 2019).
Therefore, we adopt a more integrated approach, involving the assessment of multiple ion channels' effects, that is considered more predictive and effective. Our services not only include hERG inhibition testing but also cover inhibitory assessments of potassium, calcium, and sodium channels, as well as other related channels or receptors. Additionally, we provide "Comprehensive Cardiotoxicity Assessment (CiPA)" and "Langendorff Isolated Perfused Heart Assay" services. These comprehensive and precise assessment methods enable the evaluation of your drug's in vitro cardiotoxicity potential, thereby reducing the risk of development failure due to cardiac safety issues, and consequently minimizing economic losses.
Creative Bioarray offers the following in vitro cardiotoxicity assays
(1) Cardiac Ion Channel/Receptor Inhibition Assay by Patch-clamp:
The patch-clamp technique is a powerful tool for studying ion channels in cells. It allows for the measurement of currents flowing through individual ion channels or whole cells, providing insights into their functionality and pharmacological profiles.
- hERG Inhibition Assay: This assay focuses on detecting the inhibition of the human Ether-à-go-go-Related Gene (hERG) potassium channel, a key target in assessing cardiac safety.
- Nav1.5 Peak and Late Current Assays: These assays measure the activity of the voltage-gated sodium channel Nav1.5, which is important for cardiac action potential propagation.
- hKwLQT1 (Kv7.1/mink) Inhibition: Examines the interaction with Kv7.1 channels, which play a role in cardiac repolarization.
- hKv4.3_KChIP Inhibition: Targets the Kv4.3 channels involved in the transient outward potassium current in the heart.
- GABA Receptor (a1β2y2) Inhibition: Focuses on GABA receptor ion channel modulation.
- Cav1.2 Inhibition Assay: Assesses the effect on Cav1.2 calcium channels, significant for cardiac muscle contractions.
- Kir2.1, Kv1.5, Nav1.3, Nav1.2, and Nav1.7 Inhibition Assays: Evaluate interactions with various ion channels key to cardiac and neural activities.
(2) Cardiac Ion Channel Binding Assay:
This assay involves the use of radioactive ligands to study the binding properties and kinetics between the target and the test compound.
- hERG Radioligand Binding Assay: Uses radiolabeled dofetilide to determine the binding affinities of the test compound and hERG channels.
- hNav1.7 Radioligand Binding Assay: Focuses on the binding properties relating to the Nav1.7 channel, implicated in pain sensation and nerve function.
(3) FLIPR assay:
The FLIPR (Fluorescent Imaging Plate Reader) is a screening technique that can quantify fluorescence variation as a function of ion channel activity. The method allows for the measurement of cell physiological activity in real time by identifying cells with fluorescent markers whose fluorescence intensity changes with the intracellular calcium ion concentration.
- Cav1.2 inhibition assay by FLIPR
- hERG FLIPR assays
- TRPC5, TRPV1, and hP2X3 FLIPR assays
(4) Comprehensive in vitro proarrhythmia assay (CiPA):
CiPA is a novel cardiac safety screening proposal that evaluates drug effects on each cardiac ion channel type individually (high-throughput), predicts net effect on the cardiomyocyte action potential with computer modeling, and then verifies the in silico conclusions using human stem cell-derived cardiomyocytes. Creative Bioarray Offer a full cardiotoxicity profile by examining key channels (hERG, NaV1.5, Cav1.2, Kv7.1/minK, Kv1.5, Kv4.3, Kir2.1, Kir3.1/3.4, Kir6.2/sur2, CaV3.2, HCN2, HCN4) that contribute to cardiac rhythm and action potentials.
(5) Langendorff isolated perfused heart assay:
This ex vivo system evaluates compound effects on heart function by maintaining isolated hearts in a controlled environment. It monitors cardiac parameters like heart rate, contractility, and conduction in real-time, providing holistic insights into cardiac effects.
(6) Cardiomyocyte-based assays
- Microelectrode array (MEA), a high-throughput, functional platform that can be applied to the detection of prolongation, alterations in beat rate, proarrhythmic events, and dysregulation of conduction by measuring the extracellular voltage of beating cardiomyocyte cultures.
- iPSC-derived cardiomyocytes
With 3D cardiomyocyte spheroids and high-content imaging devices, Creative Bioarray is able to provide a cardiotoxicity service that monitors the effects of compounds on cardiomyocytes at a structural and phenotypic level.
Our Service Advantages
- Utilize the latest instrumentation and methodologies to ensure the most accurate results.
- From single channel analysis to comprehensive heart evaluations, offering a complete solution for cardiac safety.
- Our highly trained scientists possess extensive experience in electrophysiology and pharmaceutical safety assessment.
- Tailor services to meet specific client needs, accommodating both small and large-scale projects.
FAQ
Q1: How do I know if my compound requires cardiotoxicity testing?
First, if the compound is a new chemical entity, particularly within drug classes known to impact the cardiovascular system, testing may be needed. The target and mechanism of action are also crucial, especially for compounds that affect ion channels or cardiovascular pathways. Furthermore, if preliminary in vitro or toxicological studies indicate potential cardiotoxic effects, more in-depth testing should be conducted. Compliance with regulatory requirements (such as ICH S7B for non-clinical testing and ICH E14 for clinical evaluation) is essential. Additionally, if similar compounds have shown cardiotoxic effects or literature suggests cardiovascular risks, it warrants attention. Results from animal studies indicating changes in heart rate, blood pressure, or ECG patterns might also necessitate further cardiotoxicity investigation.
Q2: Which in vitro cardiotoxicity assay shall I choose?
At the early discovery stage, when the focus is on initial screening to identify potential cardiotoxicity among a large number of compounds, conducting an ion channel inhibition screening assay, particularly the hERG inhibition assay, is advisable for rapid evaluation. This allows for quick elimination of compounds with potential cardiac risks. Once promising candidates are identified, moving into the preclinical stage, more detailed assessments of cardiotoxicity become necessary. This stage should include experiments using iPSC-derived cardiomyocytes to gain insights into how compounds affect cardiac cells in a more physiologically relevant context. A comprehensive investigation of ion channel and receptor inhibition, as well as heart contractility assays, should be undertaken to thoroughly assess the cardiotoxic potential and ensure the safety profile of the candidate compounds before advancing to clinical trials.
Q3: What are the advantages of using iPSC-derived cardiomyocytes in cardiotoxicity testing?
iPSC-derived cardiomyocytes offer a human-relevant model that can provide more predictive results compared to traditional animal models, as they closely mimic the electrophysiological properties of human cardiac cells.
References
- Sayed N, Ameen M, et al. Personalized medicine in cardio-oncology: the role of induced pluripotent stem cell. Cardiovasc Res. 2019. 115(5):949-959.
- ICH S7B Non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals - Scientific guideline. 2005.
- E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Food and Drug Administration. 2005.
Explore Other Options

