ONLINE INQUIRY

Human Liver S9 Fraction-50 Donors
Cat.No.: CSC-C4093X
Species: Human
Source: Liver
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
1. Conduct studies of metabolic stability (% of original drug remaining over time);
2. Identify which cytochrome P450 (CYP) enzymes oxidize a drug candidate and which UDP-glucuronosyltransferase (UGT) enzymes are responsible for glucuronidation;
3. Identify, quantify and characterize drug metabolites;
4. Describe a drug's metabolic pathways;
5. Identify inhibition of cytochrome P450 (CYP) enzymes.
Human liver S9 fraction-50 donors is a mixture obtained by centrifugation of liver tissue homogenate from 50 donors. Large pieces like nuclei and mitochondria are taken from intact liver cells, with liver microsomes and cytoplasmic tissue kept intact. In contrast to liver microsome, S9 does not only contain the enzymes of the microsome, but also various cytosolic enzymes within the cytoplasm including aldehyde oxidase, xanthine oxidase, sulfonytransferase, methyltransferase, N-acetyltransferase, and glutathione transferase as well as NADPH, UDPGA, PAPS and other cofactors involved in the reactions of drug metabolism. Thus, the S9 fraction can maintain both phase I and phase II metabolic reactions and hence it offers a wider metabolic repertoire than liver microsomes. While part of the cofactor was present in the S9 fraction, it was not sufficient and exogenous cofactor would need to be added for useful purposes. For example, adding NADPH to activate the β-nicotinamide adenine dinucleotide phosphate regeneration system, adding UDPGA to activate the uridine 5'-diphosphate-α-D-glucuronidase system, adding GSH to activate glutathione transferase system, and PAPS addition to activate 3'-phosphoadenosine-5' -phosphosulphate activity. Additionally, unlike hepatocytes, the S9 fraction lacks a membrane barrier, which means that substances do not need to cross the liver cell membrane to undergo metabolic reactions. This characteristic is particularly advantageous in studies investigating inhibitor mechanisms, as it negates the need for permeability.
Over the past decade, researchers have consistently utilized liver S9 fraction as a primary system for in vitro drug screening. It offers economic and high-throughput advantages in eliminating compounds with poor metabolic stability and low success probability.
Metabolite Profiles of Six C-Glycosides in Human Liver S9 Fraction and Human Liver Microsomes.
Several medical plants belonging to the genera Passiflora, Viola, and Crataegus accumulate flavonoid C-glycosides, which likely contribute to their efficacy. But information regarding their phase I and II metabolism in the liver are lacking. Therefore, in this study, the metabolism of six flavone C-glycosides of the apigenin and luteolin types was examined using human liver microsomes (HLM) and human S9 fraction.
The metabolism of the six flavone C-glycosides showed that mono-glycosylic flavones orientin, isoorientin, vitexin, and isovitexin are metabolized by HLM (Fig. 1), while for diglycosylic flavones schaftoside and isoschaftoside, no phase I or II metabolites were detected. Analysis of the LC-MS data showed that, all six metabolites corresponding to the molecular formula C27H28O16. In comparison to isoorientin, all other mono-glycosylic flavones showed no strongly predominating glucuronidated metabolite (Fig. 1A2, C, D). And these metabolites of orientin, isoorientin, vitexin, and isovitexin are all monoglucuronides.
The metabolism with human S9 fraction showed that, for orientin, isoorientin, vitexin, and isovitexin, the same mono-glucuronidated metabolites as described above for HLM were detected, while for schaftoside and isoschaftoside, again, no phase I or II metabolites were found. For orientin and isoorientin, three additional metabolites each, orientin-S1/S2/S3 (Fig. 1B2) and isoorientin-S1/S2/S3 (Fig. 1B1), respectively, were identified, with O-S3 and IO-S3 being the strongly dominating metabolites. In parallel, the LC-MS data of these metabolites were analyzed. These results suggest that those six metabolites are all mono-sulfates. Thus, only the luteolin-based C-flavones orientin and isoorientin show mono-glucuronidated and mono-sulfated metabolites formed in human S9 fraction. Thus, it can be assumed that the ortho dihydroxy structure at C-3′/C-4′ in the B ring could be an essential structural feature for being accepted by the sulfotransferases expressed in human liver S9 fraction.
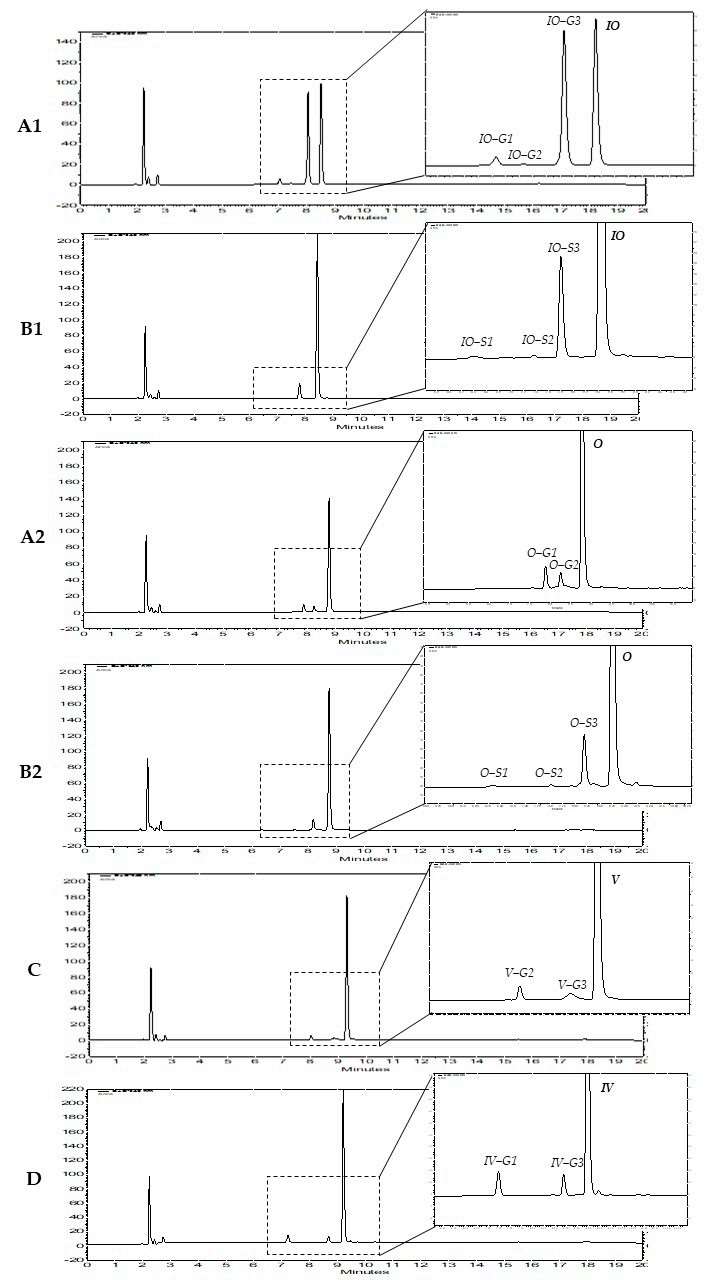 Fig. 1. HPLC chromatograms of four flavone C-glycosides and their phase II metabolites (Tremmel, M., Paetz, C., et al., 2021).
Fig. 1. HPLC chromatograms of four flavone C-glycosides and their phase II metabolites (Tremmel, M., Paetz, C., et al., 2021).
Identification of In Vitro Phase I and II Metabolites in Pooled Human Liver S9 Fraction Incubations
Lately, the two hallucinogenic 1cP-LSD and 4-AcO-DET have appeared on the global market. Knowledge about their metabolism to identify potential metabolic targets for analysis and their cytotoxic properties is lacking. This article studied their metabolism in vitro and in vivo in pooled human liver S9 fraction (pHLS9) and in zebrafish larvae (ZL) by means of liquid chromatography-high-resolution tandem mass spectrometry.
A total of seven phase I metabolites were identified after 1cP-LSD incubation with pHLS9, and no phase II metabolites were found. Analysis of the MS2 spectrum showed that A1 indicated a N-deacetylation at the N1-acyl substituent coupled with N-deethylation at the N,N-diethylamine group. N-deacetylation combined with N-demethylation on N6 led to the emergence of metabolite A2. LSD (A3) was formed after N-deacetylation of 1cP-LSD. Hydroxylation of LSD (A3) at the ergoline core resulted in the formation of 1-deacyl-hydroxy 1cP-LSD (A4). Metabolite A5 was N-deethylated, and A6 formed by N-demethylation. The hydroxy metabolite (A7) had a hydroxyl group on the N,N-diethylamine part. All phase I metabolites were identified in pHLS9 incubation both after 1 h and 6 h, except A2, which appeared only after 6 h (Fig. 2). In vitro phase II metabolism seemed to play a minor role which was in accordance with similar findings for the related LSD derivative 1 P-LSD using pHLS9.
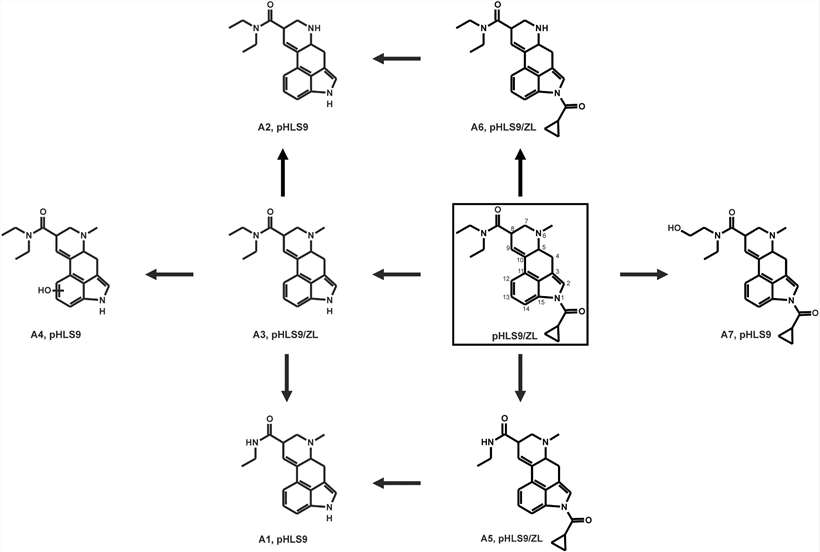 Fig. 2. Proposed in vitro and in vivo metabolic pathways of 1cP-LSD studied in pHLS9 and ZL after microinjection (Gampfer, T. M., Schütz, V., et al., 2024).
Fig. 2. Proposed in vitro and in vivo metabolic pathways of 1cP-LSD studied in pHLS9 and ZL after microinjection (Gampfer, T. M., Schütz, V., et al., 2024).
Four phase I and one phase II metabolites of 4-AcO-DET after incubation with pHLS9 were detected. Multiple metabolites of 4-AcO-DET were found in all negative controls. A higher abundance of potential metabolites in enzyme-containing incubations than in negative controls was required for positive identification. Single O-deacetylation produced 4-OH-DET (B1). Ester hydrolysis coupled with hydroxylation at the indole led to three potential O-deacyl-hydroxy isomers, with B3 showing hydroxylation at the indole core and B5 and B6 displaying different fragment patterns and retention times. The only phase II metabolite was formed by O-deacetylation followed by glucuronidation. All five metabolites were identified in pHLS9 incubations at both 1 hour and 6 hours (Fig. 3).
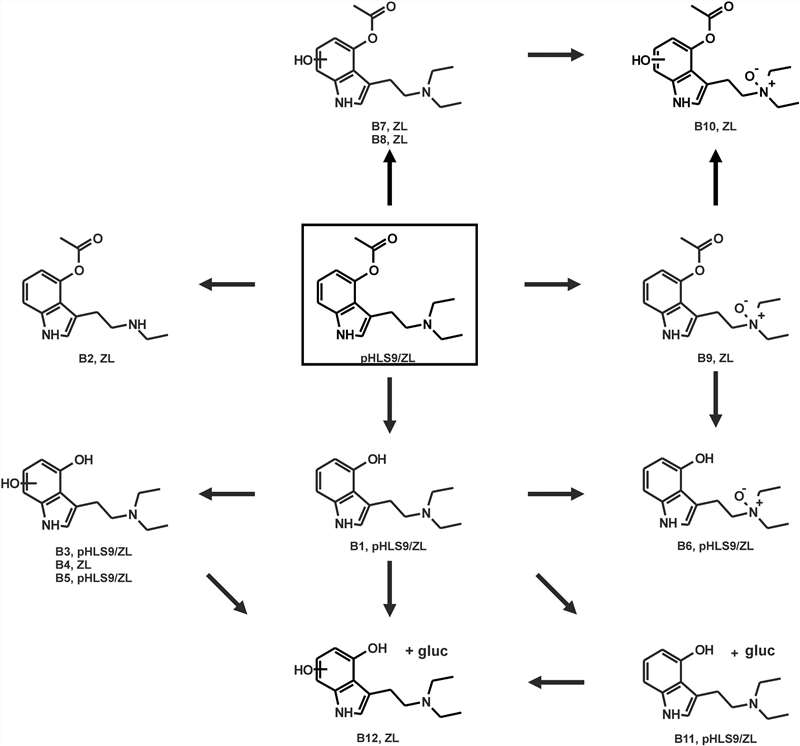 Fig. 3. Proposed in vitro and in vivo metabolic pathways of 4-AcO-DET studied in pHLS9 and ZL after exposure via immersion bath (Gampfer, T. M., Schütz, V., et al., 2024).
Fig. 3. Proposed in vitro and in vivo metabolic pathways of 4-AcO-DET studied in pHLS9 and ZL after exposure via immersion bath (Gampfer, T. M., Schütz, V., et al., 2024).
Ask a Question
Write your own review






