ONLINE INQUIRY

Human Hepatic Progenitor Cells
Cat.No.: CSC-7680W
Species: Human
Source: Liver
Cell Type: Progenitor Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Orders are delivered via Federal Express courier.
Must be processed immediately upon shipment receipt.
Media: Store at 2-8 °C
Cryopreserved cells: Liquid nitrogen
All Bioarray Inc products are for research use
Human hepatic progenitor cells (hHPCs), also known as biliary-like epithelial cells or small hepatocytes, are characterized by their oval shape and high nucleocytoplasmic ratio. They are located reside in the smallest ramifications of the biliary tree, between bile ductepithelial cells and the bile canaliculi formed between hepatocytes, possessing the potential for bidirectional differentiation into hepatocellular and biliary type cells. Typically, hHPCs originate from the canals of hering or terminal bile duct cells. hHPCs usually express markers of both biliary epithelial cells (keratin7, K19, K14) and hepatocytes lineages (K8, K18, C-met, albumin), as well as other markers like epithelial cell adhesion molecule (EpCAM), CD133, and α-fetoprotein (AFP). However, no single specific marker protein has been identified for these cells.
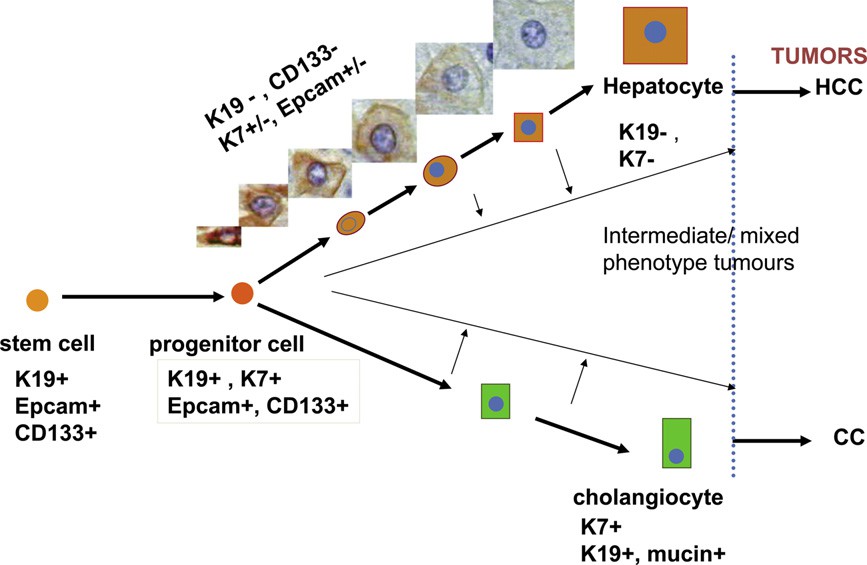 Fig. 1. Hepatic progenitor cells can differentiate into cholangiocytes and hepatocytes through intermediate cell types (Roskams, T., Katoonizadeh, A., et al., 2010).
Fig. 1. Hepatic progenitor cells can differentiate into cholangiocytes and hepatocytes through intermediate cell types (Roskams, T., Katoonizadeh, A., et al., 2010).
Under normal physiological conditions, hHPCs remain dormant. However, they become activated and differentiate into hepatocytes or bile duct cells in response to acute liver injury or when the proliferation of mature hepatocytes is inhibited. In general, the proliferation of hHPCs is positively correlated with the severity of liver damage and is regulated by a variety of signaling pathways, such as Wnt/β-catenin pathway involved in the differentiation of hHPCs into hepatocytes. While Notch signaling pathway plays a central role in the differentiation of hHPCs into bile duct cells. Other pathways, such as Hippo/YAP signaling, HGF/c-Met signaling, and TWEAK/Fn14 signaling, also regulate HPC differentiation.
The strong differentiation potential of hHPCs is closely linked to hepatocellular carcinoma. Studies have shown that chronic and long-term inflammation can lead to aberrant proliferation and differentiation of hHPCs, which may further undergo malignant transformation into cancer stem cells. Despite these risks, the remarkable proliferative ability of hHPCs is currently being investigated as a promising therapeutic alternative for patients with end-stage liver disease, offering a potential alternative to liver transplantation. Thus, understanding the mechanisms underlying the activation, differentiation, and proliferation of HPCs is of critical importance.
ERα and ERβ Promote Hepatic Growth and Differentiation through Interaction with Proteins and Regulation of Downstream Target Genes
Improving liver regeneration (LR) capacity and thereby liver function reserve is a critical bridging strategy for managing liver failure patients. The correlation between an increase of nuclear estrogen receptor (ER) with the onset of DNA synthesis in LR has been reported for decades. This paper demonstrated that estrogen signals orchestrate hepatic repopulation and differentiation viadistinct transcriptome patterns governed by Erα or ERβ.
To examine the role of ERβ in hepatic differentiation, human hepatic progenitor cells (HepRG) were treated with E2, PPT, or the ERβ-specific ligand DPN for 2 and 4 days. Hepatocyte (Alb, AFP, G6PD, GST) and cholangiocyte (KRT19, CECAM1, TMSb4x) markers, along with the liver progenitor marker HNF4α, were monitored. After 2 days, DPN significantly upregulated these markers, unlike E2 and PPT (Fig. 1c). After 4 days, only DPN showed a marked increase in expression (Fig. 1d). Moreover, knocking down Ube3a (one unique ERβ-interacting protein) reduced Ifna5 and albumin expression, while DPN treatment dramatically upregulated Ifna5 (Fig. 1e, lane 1 vs. lane 2). Ube3a knockdown also nullified DPN-induced gene expression (Fig. 1e, lane 3 vs. lane 4), indicating Ube3a's role in ERβ-specific gene expression and hepatocyte differentiation.
Overall, estrogenic signals either promote cell growth via the ERα-Chd1 axis or facilitate hepatic differentiation via the ERβ-Ube3a axis (Fig. 1). Thus, ERα and ERβ are crucial for ensuring tissue quality during liver regeneration.
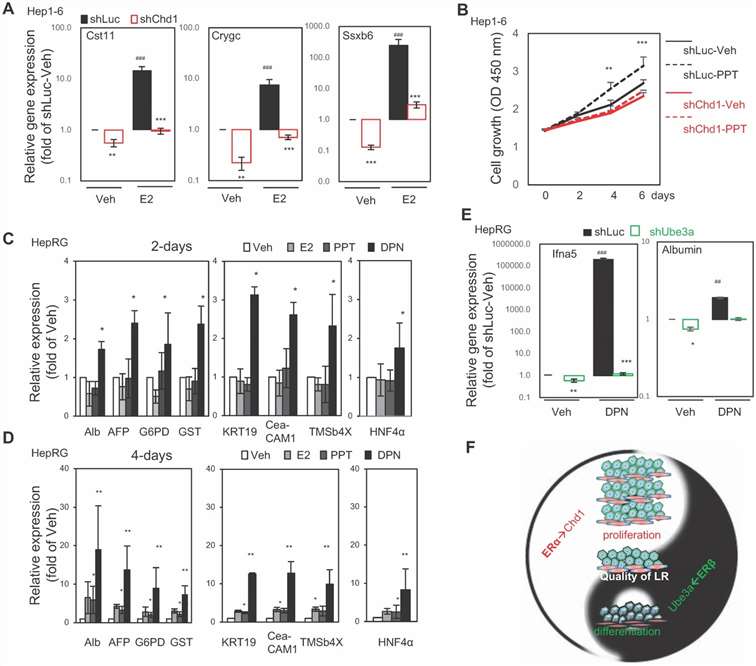 Fig. 1. The ERα→Chd1 axis for cell proliferation and the ERβ→Ube3a axis for liver function/differentiation in vitro. (Kao TL., Kuan YP., et al., 2018)
Fig. 1. The ERα→Chd1 axis for cell proliferation and the ERβ→Ube3a axis for liver function/differentiation in vitro. (Kao TL., Kuan YP., et al., 2018)
Isolation and Characterization of Extracellular Vesicles Derived from Human Hepatic Progenitor Cells
Frostbite is a cold-induced tissue injury that occurs when the temperature falls below the freezing point of tissue, and severe cases can result in amputation. Promoting deep frostbite wound healing can reduce the risk of amputation. Currently, there have been reports suggesting that stem cell-derived extracellular vesicles might aid in wound healing, but related research is limited. Zhang's team aims to investigate the potential effect of human hepatic progenitor cell-derived extracellular vesicles (EVs) on the healing of deep frostbite wounds.
Human hepatic progenitor cells (hHPCs) at passage 5 exhibited a fascicular and whorled shape (Fig. 2A). To obtain the target extracellular vesicles, cultured hHPCs were subjected to gradient centrifugation to sequentially remove cells, cell debris, and contaminating proteins. The pelleted EVs were then washed with PBS and sterilized by filtration. Then, the hHPC-derived EVs were examined by nanoparticle tracking analysis (NTA), transmission electron microscope (TEM) and Western blotting for biomarkers. As demonstrated by NTA results, the diameter of EVs ranged from 30 to 150 nm (Fig. 2B). Typical cyathiform structure was observed under the TEM scope (Fig. 2C). In addition, EVs markers, including CD9 and Alix, were also detected by Western blotting (Fig. 2D). The above results proved the successful isolation of EVs from hHPCs. Finally, the internalization ability of EVs by HSFs was examined by fluorescent dying. To this end, EVs were labeled with PKH67 (green) and co-cultured with HSFs for 4 h. Under the fluorescence microscope, green fluorescence points were detected among HSFs (DAPI, blue), which indicated that the EVs were absorbed by HSFs (Fig. 2E).
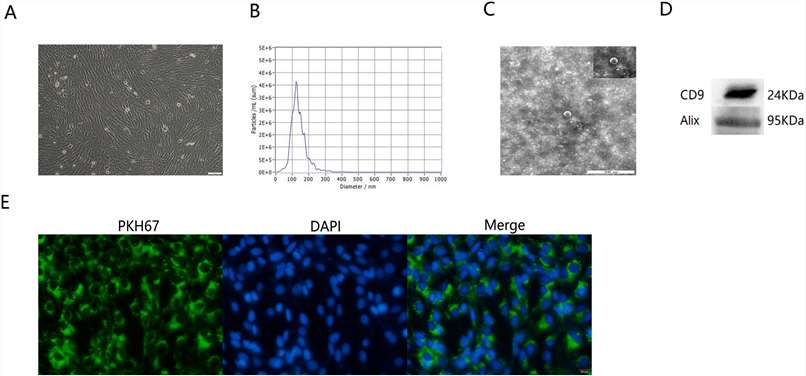 Fig. 2. Characteristics of EVs derived from hHPCs (Zhang, N., Yu, X., et al., 2022).
Fig. 2. Characteristics of EVs derived from hHPCs (Zhang, N., Yu, X., et al., 2022).
Ask a Question
Write your own review






