SAS
Cat.No.: CSC-C6334J
Species: Homo sapiens (Human)
Source: Oral Cavity; Tongue
Morphology: epithelial-like
Culture Properties: Adherent cells
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Store in liquid nitrogen.
The SAS cell line is a widely utilized model in cancer research, derived from a human squamous cell carcinoma (SCC) of the tongue. SCC is one of the most common types of cancer that originates from the squamous epithelial cells that line various organs, including the skin, mouth, throat, and lungs. In the case of the SAS cell line, the carcinoma developed in the oral cavity, specifically on the tongue, which is a frequent site for head and neck cancers.
SAS cell line recapitulates the characteristics of the original tumor. It exhibits typical squamous cell morphology, with polygonal cells that form sheets and demonstrate keratinization, a process that is characteristic of SCC. The SAS cells also express markers that are associated with squamous cell differentiation, further validating their use as a model for studying SCC.
The SAS cell line has been extensively used in research to understand the biology of SCC, including the roles of oncogenes, tumor suppressor genes, and the tumor microenvironment. It has been employed in studies focusing on tumor invasion, angiogenesis, and resistance to chemotherapy and radiation therapy. Additionally, the SAS cell line is useful for screening and evaluating new anti-cancer drugs and therapies, as it can provide insights into their mechanisms of action and potential effectiveness against SCC.
Evaluation of SAS Cell Growth and Viability after BNCT Treatment
Human tongue SCC cells (SAS) were used, which represent a type of head and neck cancer that is already treated with boron neutron capture therapy (BNCT) for both in vitro and in vivo experiments. After incubation with or in the absence of 10B-boronophenylalanine (BPA) at 25 ppm and neutron beam irradiation, survival of SAS cells was decreased in cell growth assay condition (Table 1, Fig. 1).
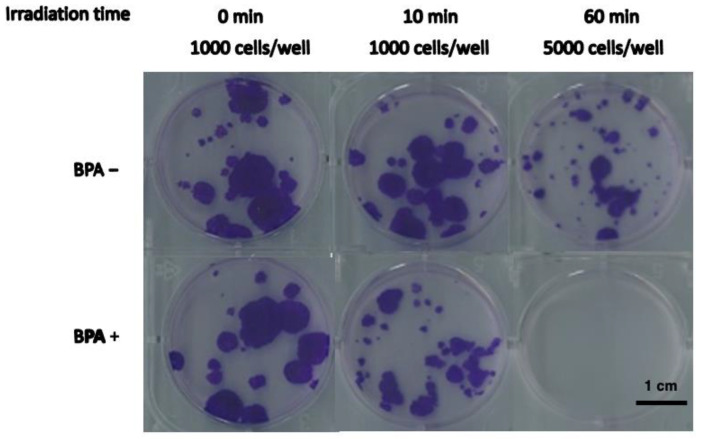 Fig. 1 Growth of SAS cells after BNCT or neutron irradiation alone. SAS cells were irradiated with neutrons (BPA−) or as BNCT (pretreatment with BPA at 25 ppm [10B]). The cell growth ratio after 8 days of culture. (Perico D, et al., 2023)
Fig. 1 Growth of SAS cells after BNCT or neutron irradiation alone. SAS cells were irradiated with neutrons (BPA−) or as BNCT (pretreatment with BPA at 25 ppm [10B]). The cell growth ratio after 8 days of culture. (Perico D, et al., 2023)
Table 1. The cell growth assay of SAS cells after BNCT treatment. (Perico D, et al., 2023)
| Irradiated time (min) | Inoculated cell numbers | Cell growth ratio | |
| BPA+ | BPA= | ||
| 0 | 1000 | 1.00 ± 0.04 | 1.00 ± 0.11 |
| 10 | 1000 | 0.40 ± 0.20 | 1.11 ± 0.15 |
| 60 | 1000 | <6.5 × 10-4 | 0.10 ± 0.04 |
Cucurbitacin E as Inducer of Cell Death and Apoptosis in SAS Cells
To explore this anti-tumor activity of cucurbitacin E (CuE) against the SAS cells, an in vitro study was initiated by treatment of the SAS cells with increasing doses of CuE (0, 1.25, 2.5, and 5 μM) for 24 h. The proliferation of these CuE-treated cancer cells was then measured by the MTT method; the results summarized in Fig. 2 indicate that the survival and proliferation of the SAS cells both decreased per increase of the dose of CuE added to the cell cultures, but not in the MRC-5 and HS68 cells (Fig. 2b) which show a dose-dependent reduction (y = 8.15x + 19.903, R2 = 0.961). Moreover, CuE was noted to induce a morphological change in the SAS cells. A microscopic examination showed that following the exposure to CuE (2.5 μM) for 6 to 24 h, the cells displayed a remarkable change in their morphology, and CuE induced the death of cancer cells, which formed a suspension in the medium.
To explore the potential role that CuE could play in the apoptosis of SAS cells, the ApopNexin FITC apoptosis detection kit has been used to identify the formation of apoptotic cells in the SAS cells after 6 h exposure to CuE. A typical set of results for the ApopNexin FITC apoptosis detection kit is illustrated in Fig. 3a, in which the annexin V-FITC deposits are indicative of the positive existence of apoptotic cells. A dose-dependent increase in apoptosis was observed (y = 12.227x + 2.2467, R2 = 0.9819), that is: the higher the dose of CuE (1.25, 2.5, and 5 μM) used in the exposure, the greater the extent of apoptosis (Fig. 3b). The increase in the percentages of apoptotic SAS cells was observed at all doses after treatment for 4 h. In 4 h, approximately 12.44 ± 0.92% of SAS cells were apoptotic (early apoptosis and late apoptosis) relative to cells in CuE 0 μM. The rate of apoptotic SAS cells increased to 29.38% ± 2.97% with 1.25 μM CuE treatments. When the concentrations of CuE increased to 2.5 and 5 μM, the percentages of total apoptotic CRC cells increased to 39.65% ± 1.34% and 49.77% ± 4.04% respectively. Taken together, the observations imply that the apoptosis of SAS cells is significantly elevated by CuE.
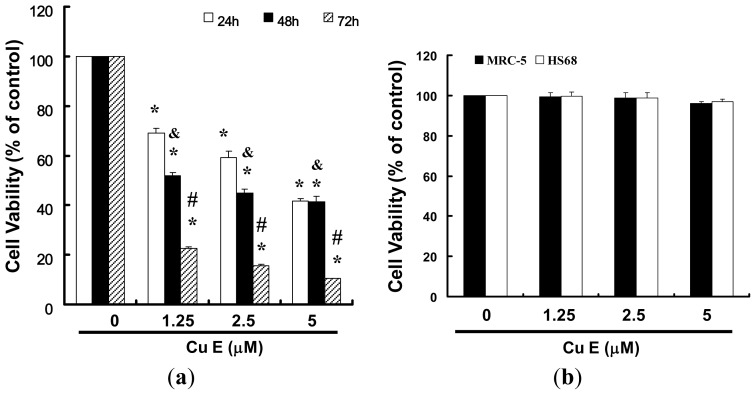 Fig. 2 CuE mediates the survival of SAS and normal fibroblast (MRC-5; normal lung fibroblasts and HS68; normal skin fibroblasts) cells, thereby inhibiting the proliferation of SAS cells. (Hung CM, et al., 2013)
Fig. 2 CuE mediates the survival of SAS and normal fibroblast (MRC-5; normal lung fibroblasts and HS68; normal skin fibroblasts) cells, thereby inhibiting the proliferation of SAS cells. (Hung CM, et al., 2013)
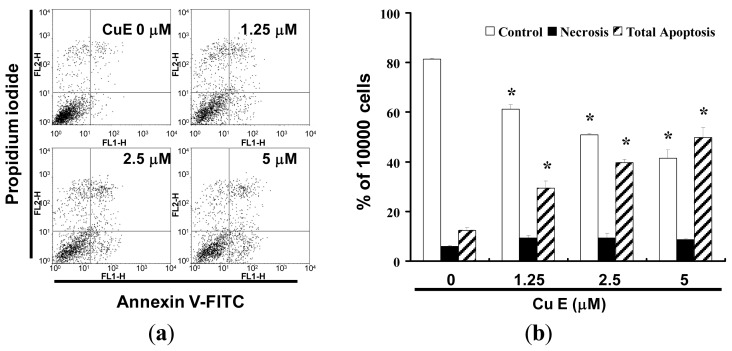 Fig. 3 (a) Influence of CuE on apoptosis in SAS cells; and (b) Total apoptosis in SAS cells after 4 h of incubation with CuE. (Hung CM, et al., 2013)
Fig. 3 (a) Influence of CuE on apoptosis in SAS cells; and (b) Total apoptosis in SAS cells after 4 h of incubation with CuE. (Hung CM, et al., 2013)
Ask a Question
Write your own review
- You May Also Need
- Adipose Tissue-Derived Stem Cells
- Human Neurons
- Mouse Probe
- Whole Chromosome Painting Probes
- Hepatic Cells
- Renal Cells
- In Vitro ADME Kits
- Tissue Microarray
- Tissue Blocks
- Tissue Sections
- FFPE Cell Pellet
- Probe
- Centromere Probes
- Telomere Probes
- Satellite Enumeration Probes
- Subtelomere Specific Probes
- Bacterial Probes
- ISH/FISH Probes
- Exosome Isolation Kit
- Human Adult Stem Cells
- Mouse Stem Cells
- iPSCs
- Mouse Embryonic Stem Cells
- iPSC Differentiation Kits
- Mesenchymal Stem Cells
- Immortalized Human Cells
- Immortalized Murine Cells
- Cell Immortalization Kit
- Adipose Cells
- Cardiac Cells
- Dermal Cells
- Epidermal Cells
- Peripheral Blood Mononuclear Cells
- Umbilical Cord Cells
- Monkey Primary Cells
- Mouse Primary Cells
- Breast Tumor Cells
- Colorectal Tumor Cells
- Esophageal Tumor Cells
- Lung Tumor Cells
- Leukemia/Lymphoma/Myeloma Cells
- Ovarian Tumor Cells
- Pancreatic Tumor Cells
- Mouse Tumor Cells