Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
FaDu
Cat.No.: CSC-C9379L
Species: Homo sapiens, human
Source: Pharynx
Morphology: epithelial
Culture Properties: Monolayer
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
vWA: 15,17
FGA: 25
Amelogenin: X
TH01: 8
TPOX: 11
CSF1P0: 12
D5S818: 12
D13S317: 8,9
D7S820: 11,12
Shipping Condition: Room Temperature
The FaDu cell line is an established human cancer cell line originally derived from a squamous cell carcinoma of the hypopharynx in a patient from Calcutta, India. It was established in the early 1970s and has since served as a valuable experimental model for studying head and neck cancers, particularly squamous cell carcinomas. As a well-characterized cell line, FaDu possesses distinct biological and morphological characteristics that enable researchers to examine various aspects of cancer biology, including tumorigenesis, metastasis, and responses to therapy. FaDu cells are often used in conjunction with various treatments to assess drug resistance and sensitivity, making them a significant tool in oncological research.
Furthermore, the FaDu cell line has been pivotal in understanding the molecular and genetic drivers of squamous cell carcinoma. Researchers have utilized it to investigate gene expression profiles, signaling pathways, and the effects of targeted therapies. Its origin from a patient with a specific type of cancer adds to its relevance, providing insights into the biology of head and neck tumors and potentially leading to the development of more effective treatment strategies.
Lovastatin Arrested Cell Cycle and Induced Apoptosis in FaDu Cells
MTT assay was employed to determine whether FaDu cell viability is altered in the presence of lovastatin. As shown in Fig. 1a, lovastatin concentration-dependently decreased FaDu cell viability after 24 h exposure. Longer exposure to lovastatin (48 h) further decreased FaDu cell viability (Fig. 1a). To determine whether lovastatin-decreased FaDu cell viability was a result of cell cycle arrest or apoptosis, flowcytometry was used. As shown in Fig. 1b, the percentage of propidium iodide (PI)-stained cells in the S region was significantly decreased in FaDu cells after exposure to lovastatin for 24 h. In addition, lovastatin increased the percentage of PI-stained cells in the G0/G1 region (Fig. 1b). Moreover, 24 h treatment of lovastatin only slightly induced cell apoptosis (sub-G1 region) (Fig. 1b). However, lovastatin significantly induced apoptosis in FaDu cells after 48 h exposure of lovastatin (Fig. 1c). To detect apoptosis in FaDu cells exposed to lovastatin, flowcytometry with PI and annexin V-FITC double-labeling was also employed. As shown in Fig. 1d, lovastatin increased the percentage of early apoptotic cells (annexin V+PI− cells) and advanced apoptotic cells and/or necrotic cells (annexin V+PI+ cells) after 48 h exposure. As shown in Fig. 1e, lovastatin increased the cleaved (active) form of caspase 3 and PARP, a selective caspase 3 substrate. These findings suggest that lovastatin induced apoptosis and inhibited cell proliferation in FaDu cells.
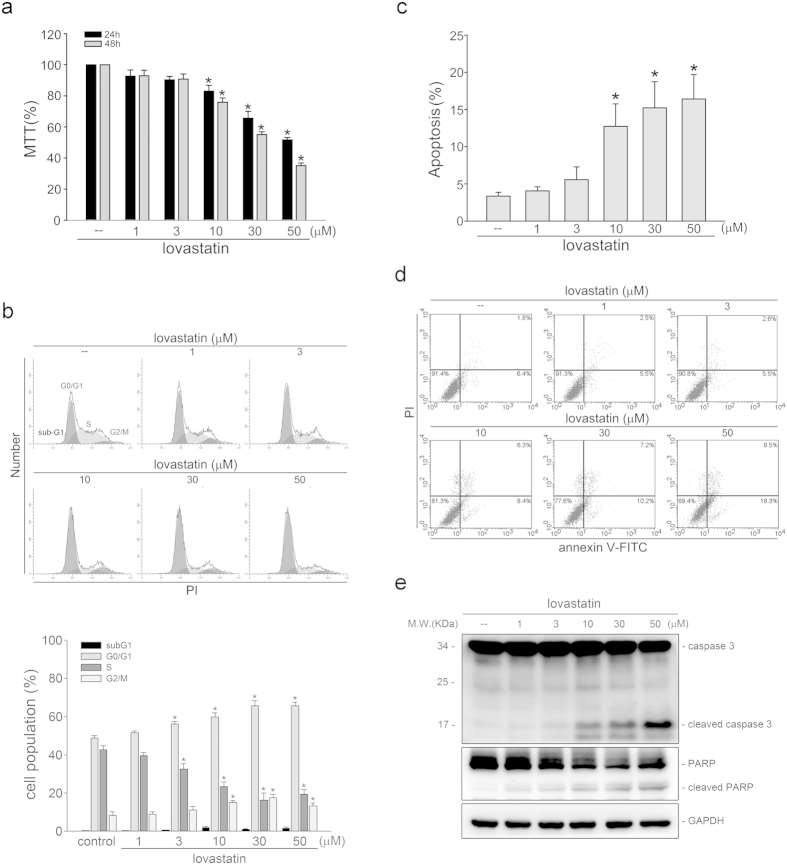 Fig. 1 (a) After treatment with indicated concentrations of lovastatin for 24 or 48 h, an MTT assay was used to determine cell viability. (b) After treatment with indicated concentrations of lovastatin for 24 h, flow cytometric analysis was used to analyze the cell cycle distribution. (c) After treatment with indicated concentrations of lovastatin for 48 h, flow cytometric analysis was used to determine the extent of cell apoptosis (subG1 region). (d) Cells were treated as in (c). Flow-cytometric analysis with propidium iodide (PI) and annexin V-FITC double staining was used to determine the extent of cell apoptosis. (e) After treatment as in (c), immunoblotting was then used to determine the cleavage caspase 3 and PARP levels. (Yen CS, et al., 2016)
Fig. 1 (a) After treatment with indicated concentrations of lovastatin for 24 or 48 h, an MTT assay was used to determine cell viability. (b) After treatment with indicated concentrations of lovastatin for 24 h, flow cytometric analysis was used to analyze the cell cycle distribution. (c) After treatment with indicated concentrations of lovastatin for 48 h, flow cytometric analysis was used to determine the extent of cell apoptosis (subG1 region). (d) Cells were treated as in (c). Flow-cytometric analysis with propidium iodide (PI) and annexin V-FITC double staining was used to determine the extent of cell apoptosis. (e) After treatment as in (c), immunoblotting was then used to determine the cleavage caspase 3 and PARP levels. (Yen CS, et al., 2016)
Upregulated MiR-107 Inhibits FaDu Cell Proliferation
To expound the role of miR-107 in HSCC, the FaDu cell line was selected, and miR-107 overexpression was achieved by miR-107 mimics transfection. Following that, cell viability was lowered by miR-107 mimics at 48 h and 72 h after transfection (Fig. 2a). Moreover, FCM results demonstrated that miR-107 overexpression increased the proportion of FaDu cells in the G1 phase of the cell cycle, but reduced the proportion of cells in the S phase (Fig. 2b). In addition, the protein expressions of cyclin D1 and CDK4 related to the cell cycle were assessed. Western blot results revealed that cyclin D1 and CDK4 levels were reduced by miR-107 overexpression (Fig. 2c, d). These findings indicated that upregulated miR-107 lowered cell proliferation possibly through the arrest of the cell cycle.
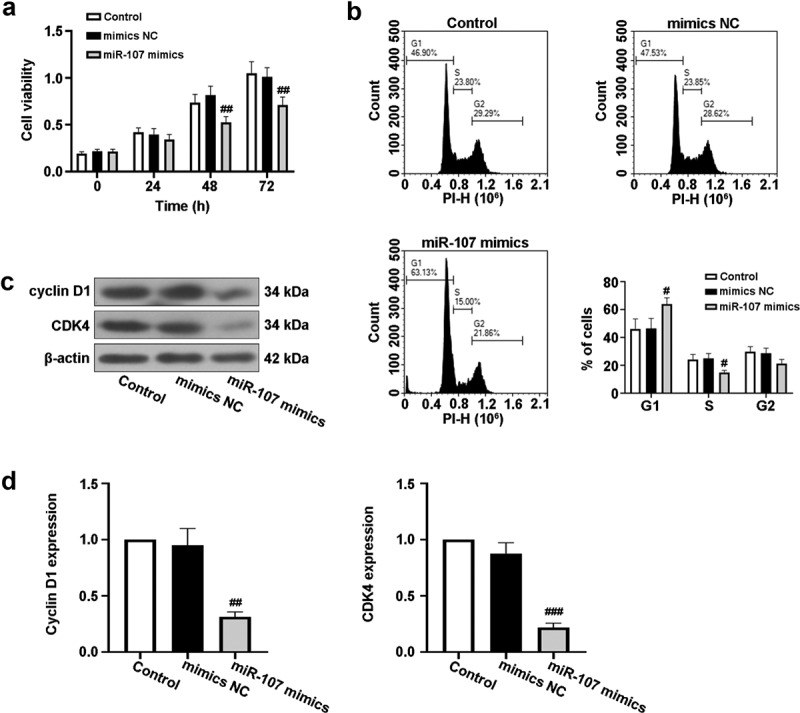 Fig. 2 MiR-107 overexpression inhibited proliferation and arrested cell cycle in FaDu cells. (a) After transfection for 24 h 48 h or 72 h, cell viability was detected by CCK-8 assay. (b) After transfection for 48 h, the cell cycle was evaluated via a flow cytometer (FCM). (c, d) After transfection for 48 h, the protein expressions of cyclin D1 and CDK4 were assessed using Western blot. (Gao X, et al., 2022)
Fig. 2 MiR-107 overexpression inhibited proliferation and arrested cell cycle in FaDu cells. (a) After transfection for 24 h 48 h or 72 h, cell viability was detected by CCK-8 assay. (b) After transfection for 48 h, the cell cycle was evaluated via a flow cytometer (FCM). (c, d) After transfection for 48 h, the protein expressions of cyclin D1 and CDK4 were assessed using Western blot. (Gao X, et al., 2022)
The optimal concentration of L-glutamine for cell culture is dependent upon the cell type and medium used but generally falls in the range of 2-6 mM.
Ask a Question
Average Rating: 4.0 | 1 Scientist has reviewed this product
Good compatibility
The tumor cell product's compatibility with various experimental techniques has allowed us to perform detailed phenotypic and functional characterizations with ease and accuracy.
10 May 2023
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need