ONLINE INQUIRY
Human Hepatocytes
Cat.No.: CSC-C1495
Species: Human
Source: Liver
Cell Type: Hepatocyte
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
HH are isolated from human liver. HH are cryopreserved immediately after purification and delivered frozen. Each vial contains >1 x 10^6 cells in 1 ml volume. HH are characterized by immunofluorescent method with antibodies to albumin, cytokeratin-18 and vimentin. HH are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. HH is not recommend for expanding or long term cultures since the cells do not proliferate in culture.
The liver is the main metabolic, detoxifying and largest visceral organ in the human body. During embryonic and liver development, some of these hepatoblasts are differentiate into hepatocytes, which are then further specialized into mature hepatic cells. Human hepatocytes (HHs) in our liver are polygonal, ranging in size from 20-30 micrometers, with 6-8 sides, which might differ in size depending on our physiological environment. They are the most common parenchymal cells in the liver, which accounts for about 70 to 85 per cent of its volume. HHs are responsible for the metabolism of carbohydrates, lipids, and proteins, detoxification of drugs, alcohol. And metabolic waste products, secretion of plasma proteins and bile, and storage of glycogen, lipids, vitamins, and minerals. During this phase, human liver cells are expressing liver markers including alpha-fetoprotein (AFP), albumin (ALB) and cytokeratins (CK-18 and CK-19).
Because they are regenerative, and repair scar tissue after relatively limited injury or surgery, HHs are becoming an important target for research into extracorporeal liver transplantation. They detoxify, making them invaluable for measuring drug clearance and toxicity. Hepatocytes can also provide a complete metabolic enzyme system, including phase I and phase II metabolic enzymes, widely used to monitor the metabolic stability and metabolism of drug candidates. Further, HHs contribute to immune responses and liver disease, assisting in antigen presentation, immune control, antiviral activity and impacting inflammation and healing with regenerative capacities. Hence, it is extremely important to create in vitro hepatocyte models.
 Fig. 1. Primary human hepatocyte cells (Jeschke, M. G., Klein, D., et al., 2008).
Fig. 1. Primary human hepatocyte cells (Jeschke, M. G., Klein, D., et al., 2008).
Wnt/β-catenin and NFκB Signaling Synergize to Trigger Growth Factor-free Regeneration of Adult Primary Human Hepatocytes
In rodents, growth factor signaling is required for hepatocytes to re-enter the cell cycle after damage, but this study identified Wnt/β-catenin signaling activation as the primary signal for proliferation in adult primary human hepatocytes.
The study found that reducing TGFβ in primary human hepatocytes (PHHs) and its activation during spheroid aggregation prevented Wnt/β-catenin or growth factor-induced cell cycle re-entry (Fig. 1A and B). However, TGFβ inhibition alone was insufficient to induce quiescent PHH proliferation, even with Wnt/β-catenin activation (Fig. 1C). IL6 and TNFα could not induce cell cycle entry alone or with Wnt/β-catenin activation and TGFβ inhibition (Fig. 1D-F). Combining cytokines with TGFβ inhibition and Wnt/β-catenin activation led to extensive proliferation, without external growth factors. This proliferation was independent of growth factor receptor signaling. Co-culturing PHH with primary Kupffer cells (KCs) significantly increased PHH proliferation (Fig. 1H). The study concluded that human KCs produce molecular cues, such as cytokines, GFs, and Wnt ligands, which prime and activate human hepatocyte proliferation.
To assess the cytokine specificity in cell cycle stimulation, the study compared pro- and anti-inflammatory cytokines. Only pro-inflammatory cytokines IL1β and TNFα showed effects (Fig. 2A). Maximum PHHs cell cycle entry and S-phase increase occurred between days 2 and 3 (Fig. 2B and C). EdU data and phosphorylated histone H3 staining confirmed active mitosis peaks early (Fig. 2D and E). The NF-κB inhibitor BAY-11-7082 halted the proliferative response (Fig. 2F). Knock-down of RELA (NFκB transducer) resulted in reduced phosphorylation of its encoded NFκB-signaling transducer p65 and decreased PHH cell cycle re-entry (Fig. 2F). Phosphoproteomic analysis showed distinct signaling networks (Fig. 2G), while cytokine treatments highlighted NF-κB regulator IKBKB and Wnt inhibitor NDRG2 phosphorylation, suggesting different signaling under GF-free conditions (Fig. 2H-J).
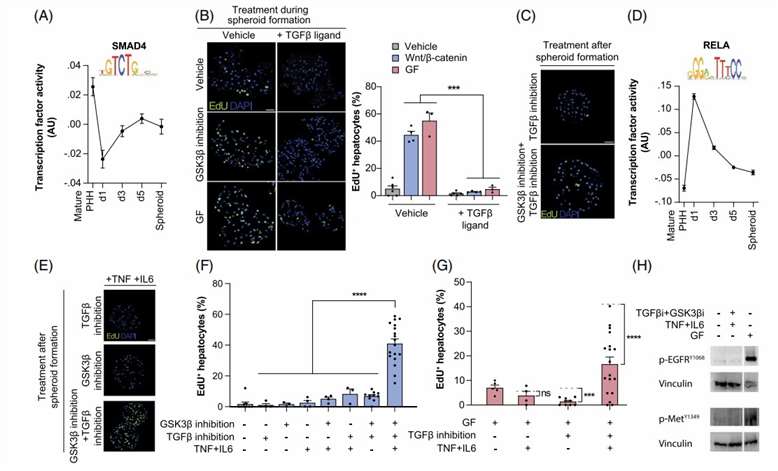 Fig. 1. Activation of cytokines and inhibition of TGFβ signaling renders primary human hepatocytes susceptible to Wnt/β-catenin-mediated proliferation (Oliva-Vilarnau N., Beusch CM., et al., 2024).
Fig. 1. Activation of cytokines and inhibition of TGFβ signaling renders primary human hepatocytes susceptible to Wnt/β-catenin-mediated proliferation (Oliva-Vilarnau N., Beusch CM., et al., 2024).
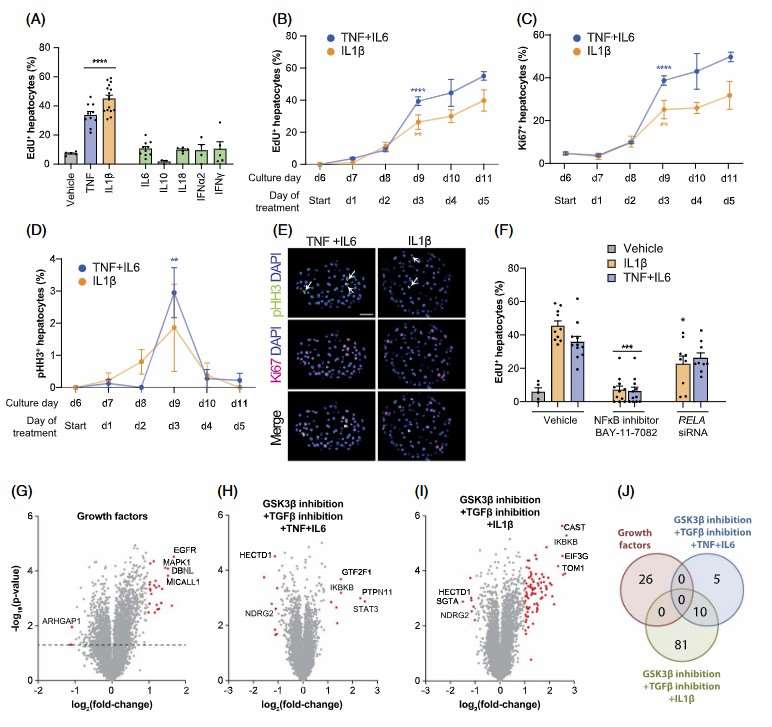 Fig. 2. Stimulation of hepatocyte regeneration requires canonical NFκB signaling (Oliva-Vilarnau N., Beusch CM., et al., 2024).
Fig. 2. Stimulation of hepatocyte regeneration requires canonical NFκB signaling (Oliva-Vilarnau N., Beusch CM., et al., 2024).
LXR Agonists Reduce Intracellular HBV RNA and DNA Levels but Have no Effect on cccDNA Levels in PHHs
The liver X receptors (LXRs), which belong to the superfamily of nuclear hormone receptors, are important physiological regulators of lipid and cholesterol metabolism. However, the association between the LXR pathway and HBV infection remains largely unclear.
To assess the effects of LXR agonists on HBV replication, synthetic agonists T0901317 and GW3965 were tested in HBV-infected differentiated HepaRG cells (Fig. 3A). HBV-infected dHepaRG cells were treated with the compounds for 9 days, reducing HBsAg, HBeAg, and HBV DNA levels dose-dependently without obvious cytotoxicity (Fig. 3B). Similarly, LXR agonists were applied to HBV-infected PHHs, where both compounds also reduced viral markers dose-dependently, showing stronger anti-HBV activity in PHHs than dHepaRG cells (Fig. 3C). Immunofluorescence staining showed that LXR agonists significantly reduced intracellular HBcAg levels in HBV-infected PHHs, with the anti-HBV activity of T0901317 compromised when LXR expression was knocked down, indicating LXR involvement (Fig. 3D).
To understand the anti-HBV effect of LXR agonists, HBV RNA levels were assessed in HBV-infected PHHs after T0901317 and GW3965 treatment. Quantification showed substantial reductions in total HBV RNA and pg/pcRNA levels using the QuantiGene 2.0 assay and HBV RNA FISH method (Fig. 4A and B). Additionally, both LXR agonists significantly decreased intracellular HBV DNA in a dose-dependent manner, while cccDNA levels remained unchanged (Fig. 4C). Nascent HBV mRNAs, measured after BU labeling, also significantly reduced following LXR agonist treatment, similar to the effects of RO8191, an HBV transcription inhibitor (Fig. 4D). These findings indicate that LXR agonists inhibit cccDNA transcription and lower intracellular HBV RNA, contributing to reduced HBV antigen and DNA production.
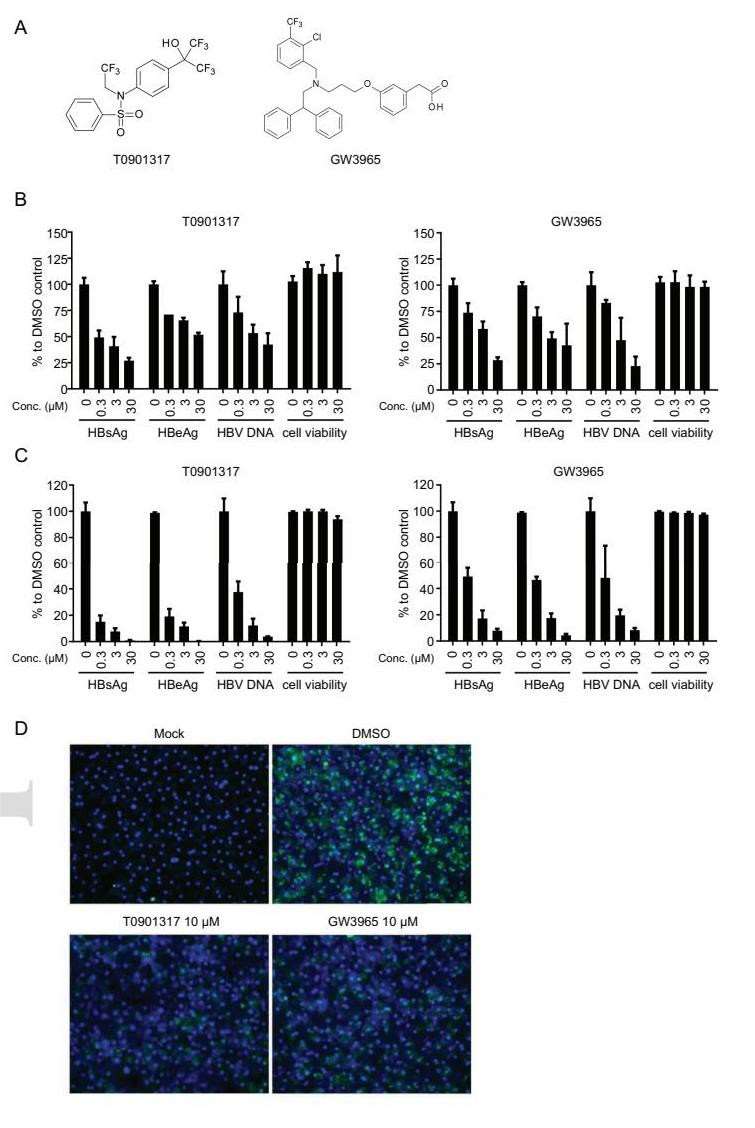 Fig. 3. LXR agonists inhibit HBV DNA replication and antigen release in both dHepaRG cells and PHHs (Zeng, J., Wu, D., et al., 2020).
Fig. 3. LXR agonists inhibit HBV DNA replication and antigen release in both dHepaRG cells and PHHs (Zeng, J., Wu, D., et al., 2020).
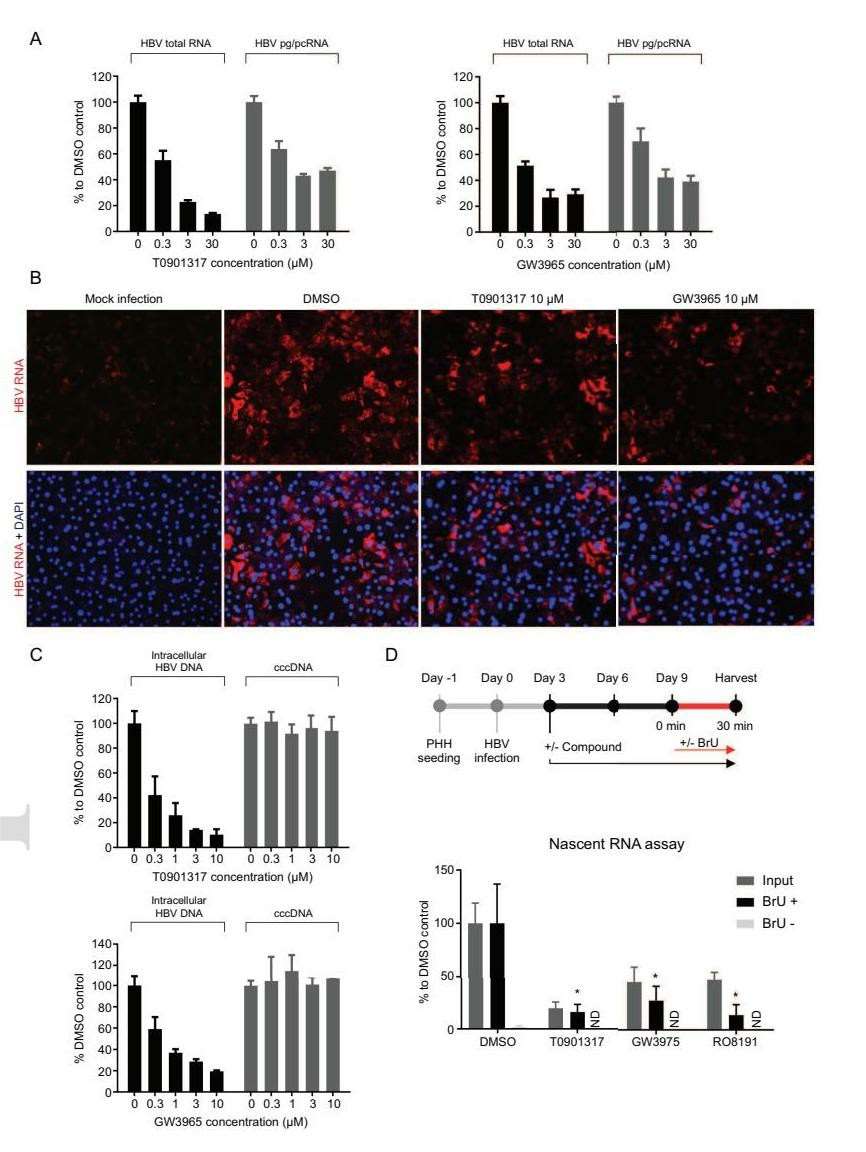 Fig. 4. LXR agonists decrease HBV RNA levels in PHHs but have no effects on cccDNA stability (Zeng, J., Wu, D., et al., 2020).
Fig. 4. LXR agonists decrease HBV RNA levels in PHHs but have no effects on cccDNA stability (Zeng, J., Wu, D., et al., 2020).
Ask a Question
Write your own review