ONLINE INQUIRY

- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Hepatocytes (HCs) and non-parenchymal cells (NPCs) constitute the liver, with parenchymal cells making up about 60-70% and non-parenchymal cells around 20-30%. Non-parenchymal cells include liver sinusoidal endothelial cells (LSECs, about 50%), lymphocytes (about 25%), Kupffer cells (KCs, about 20%), biliary cells (about 5%) and hepatic stellate cells (HSCs) (about 1%). HCs (hepatocytes) are the metabolic and hormonal regulators of the healthy liver; NPCs are responsible for the liver's structure, molecular transportation and interaction with the immune system. Among NPCs, LSECs also known as fenestrated endothelial cells, are primarily involved in substance exchange between the liver and blood. KCs are considered resident liver macrophages, which are involved in the elimination of endotoxins, microbes, dead cells and other matter that is circulating. They are also diffused in the parenchyma and portal tracts as are other innate immune cells like natural killer (NK) cells, NKT cells and γδ T cells. HSCs are located in the space of disse between hepatocytes and sinusoids, and are not active under normal physiological conditions. When injured, they get activate and turn into myofibroblasts.
They also communicate between themselves by secreting soluble molecules (cytokines, chemokines) and maintaining liver homeostasis, as well as collectively reacting to infection or toxic insult. In alcohol-related liver disease, for example, NPCs can be targeted or stimulated when damage-related molecular pattern is released. Therefore, in vitro models incorporating HC and NPC compartments is necessary to unravel the intercellular dynamics of liver biology and pathology.
Functionality of Primary Hepatic Non-parenchymal Cells in A 3D Spheroid Model and Contribution to Acetaminophen Hepatotoxicity
Bell's team developed a 3D co-culture system with primary human hepatocytes (PHHs) and non-parenchymal cells (NPCs) to investigate acetaminophen-induced liver toxicity.
Spheroids were treated with the model hepatotoxicant acetaminophen (APAP) and the response was measured using various readouts. NPCs provided protection against APAP-induced toxicity, requiring higher concentrations of APAP to reduce cellular ATP levels (Fig. 1a and b). Sensitivity differences became noticeable after 24/72 hours of treatment and increased threefold after 14 days. Additionally, cellular glutathione depletion was greater in hepatocyte-only spheroids, suggesting higher sensitivity to APAP-induced oxidative stress (Fig. 1b and e). The hepatotoxicity of APAP is linked to the formation of the reactive metabolite NAPQI (N-acetyl-p-ben-zoquinone). LC-MS/MS analysis showed that Mono- and co-culture produced similar amounts of glucuronide and sulfate metabolites, but less APAP-GSH conjugate was detected in NPC-containing spheroids. Even though both types of spheroids had the same number of hepatocytes, NPC-containing spheroids showed lower expression of APAP-metabolizing enzymes (Fig. 1d). To determine if the difference was due to varying expression of enzymes involved in APAP metabolism, mRNA levels of CYP1A2, 2E1, and 3A4 were examined. These enzymes were lower in NPC-containing spheroids despite similar albumin levels. APAP treatment further reduced these enzymes, especially in PHH spheroids where their initial levels were higher. Nrf-2 target genes Nqol and Srxnl were upregulated by APAP in both models, suggesting that oxidative stress was induced in both models (Fig. 2).
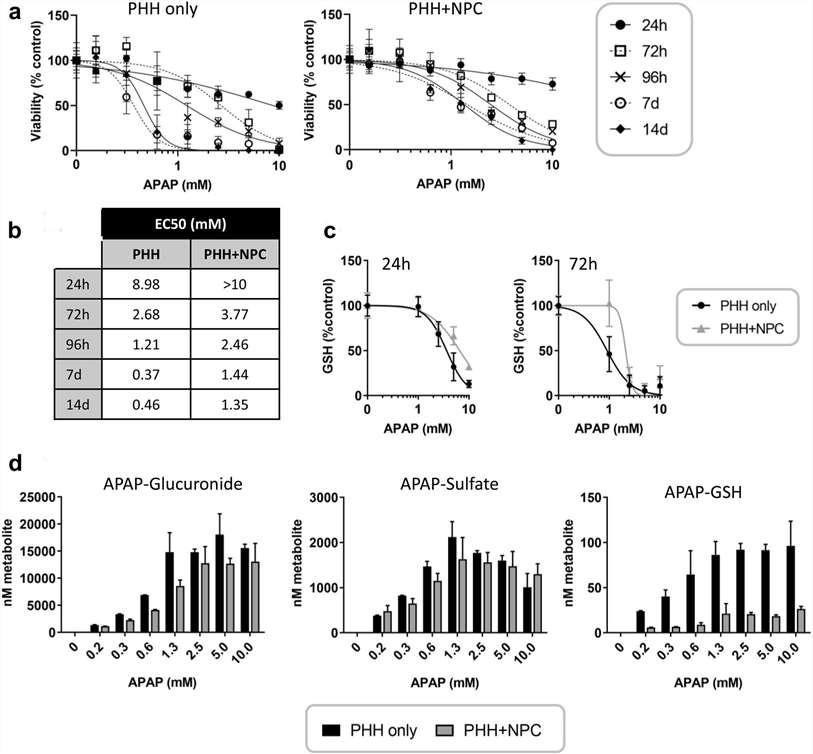 Fig. 1. Hepatic non-parenchymal cells protect against acetaminophen toxicity (Bell C. C., Chouhan B., et al., 2020).
Fig. 1. Hepatic non-parenchymal cells protect against acetaminophen toxicity (Bell C. C., Chouhan B., et al., 2020).
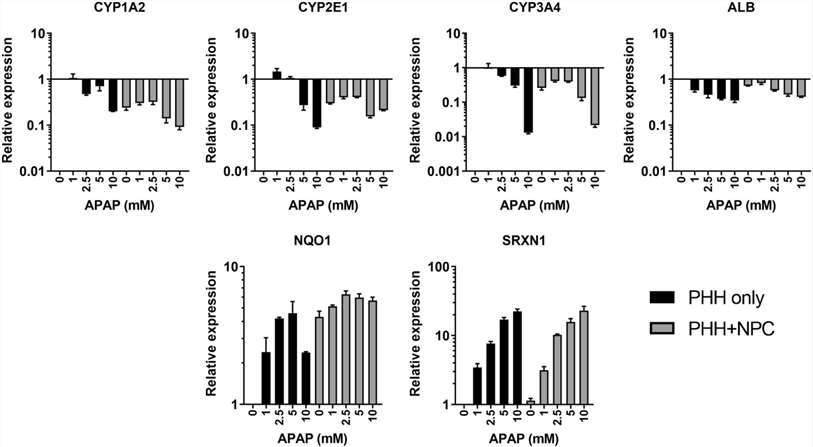 Fig. 2. Acetaminophen induces changes in cytochrome P450 enzyme expression and markers of oxidative stress (Bell C. C., Chouhan B., et al., 2020).
Fig. 2. Acetaminophen induces changes in cytochrome P450 enzyme expression and markers of oxidative stress (Bell C. C., Chouhan B., et al., 2020).
miRNAs can be sensitive and specific biomarkers of drug-induced toxicity. This study examined six miRNAs: five linked to liver injury from APAP poisoning and one linked to inflammation. Elevated levels of liver-enriched miRNAs in plasma indicate hepatocyte damage and leakage of cellular contents. Among the six miRNAs, only miR-122 was consistently detected in media samples from APAP-treated spheroids, with higher levels in PHH-only spheroids compared to those with NPCs, though only at the highest APAP concentrations (Fig. 3a). Cellular levels of miR-122 were unaffected (Fig. 3b). Neither miR-151 nor miR-885 were detected in media samples, likely due to detection limits and lower cellular expression compared to miR-122 (Fig. 3b). miR-155 and miR-382 levels were higher in NPC-containing spheroids, with miR-155 levels sixfold higher at baseline (Fig. 3b).
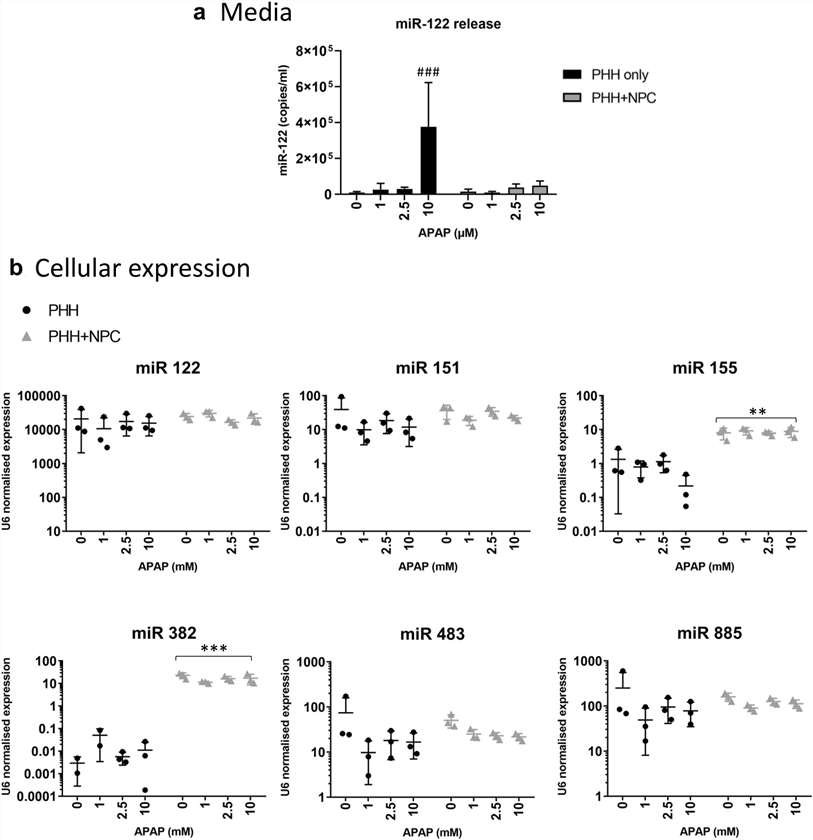 Fig. 3. Changes in miRNA expression and release following APAP treatment are influenced by the presence of hepatic non-parenchymal cells (Bell C. C., Chouhan B., et al., 2020).
Fig. 3. Changes in miRNA expression and release following APAP treatment are influenced by the presence of hepatic non-parenchymal cells (Bell C. C., Chouhan B., et al., 2020).
Characterization of Non-parenchymal Cell Population in a 3D Human Liver Model
Moss's team employs a robotic biomanufacturing platform and off-the-shelf components to automate the fabrication of vascularized human liver tissue models. These liver tissue models are constructed using primary hepatocytes, a crude preparation of non-parenchymal liver cells and isolates of fragmented adipose microvessels (haMVs) to supplement the tissue environment. Primary hepatocytes are considered the gold standard for assessing hepatotoxicity in vitro. However, the inclusion of liver non-parenchymal cells (NPCs) and endothelial cells within liver tissue models provides a more reliable platform for toxicity evaluation. Additionally, the included microvessel fragments can spontaneously organize into vascular structures within a 3D environment, enhancing the phenotypic adaptation of the tissue environment.
The key point of adding NPCs to 3D models is that the expanded culture of isolated NPC subsets, such as sinusoidal endothelial cells, Kupffer cells, and stellate cells, which are crucial for maintaining long-term liver cultures and supporting hepatocyte function. Therefore, they analyzed engineered liver tissues for the presence of these cells using markers UEA-1, aSMA, and CD45, comparing tissues with and without human microvessels (haMVs) (Fig. 4A and B). In constructs without haMVs, few ECs (UEA-1+) were observed, but their numbers significantly increased when haMVs were included, where they were found both dispersed and clustered in microvessel-like structures (Fig. 4C and D). Alpha-actin-positive cells, likely perivascular cells, also showed a notable increase in haMV-containing constructs, often associated with the haMVs, with some dispersed throughout the tissue (Fig. 4C). CD45+ immune cells were similarly elevated, reflecting the addition of endothelial and perivascular cells with haMVs (Fig. 4E and F). The inclusion of mesenchymal cells, which are known for their pro-healing and homeostatic properties, likely enhances the stability of the tissue environment, preserving cell numbers, including hepatocytes and non-parenchymal cells (NPCs). Furthermore, cells from the microvessel fragments in 3D culture secrete growth factors and cytokines that influence cell function, which may explain the lower LDH levels observed in static cultures with haMVs, indicating reduced cell damage. Overall, the inclusion of haMVs improved the function and stability of the fabricated human liver tissues.
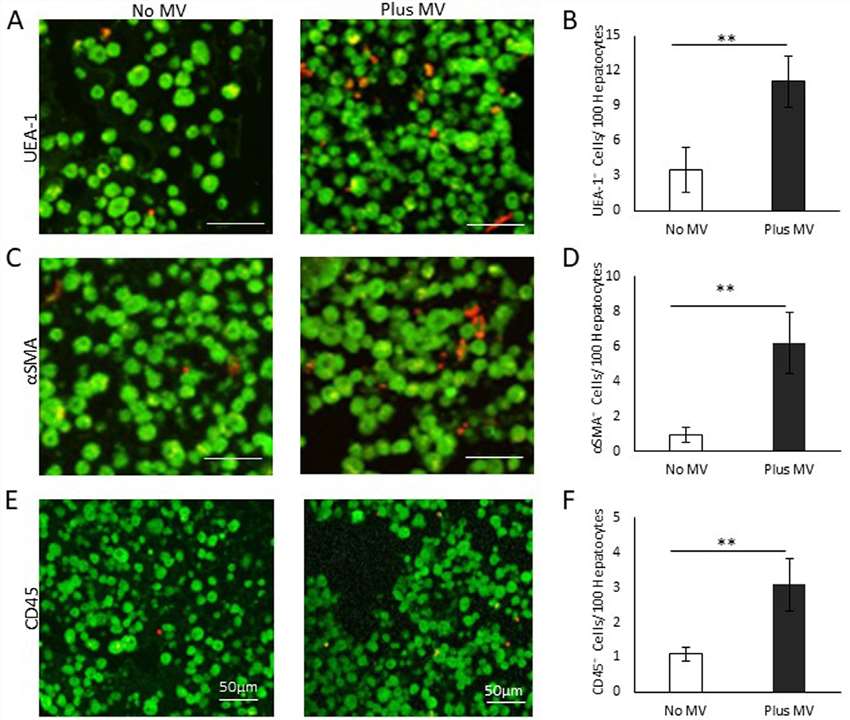 Fig. 4. NPC composition of liver tissue constructs (Moss, S. M., Schilp, J., et al., 2022).
Fig. 4. NPC composition of liver tissue constructs (Moss, S. M., Schilp, J., et al., 2022).
Ask a Question
Write your own review






