Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
MEC-2
Cat.No.: CSC-C0511
Species: Human
Source: chronic B cell leukemia
Morphology: polymorphic cells growing in suspension in large clumps; clumps can easily be segregated
Culture Properties: suspension
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
- Documents
Immunology: CD3 -, CD10 -, CD13 -, CD19 +, CD20 +, CD34 -, CD37 +, cyCD79a +, CD80 +, CD138 +, HLA-DR +, sm/cyIgG -, sm/cyIgM +, sm/cykappa +, sm/cylambda -
Viruses: PCR: EBV +, HBV -, HCV -, HIV
MEC-2 cells are an important cell line that was established in 1994 from the peripheral blood (PB) of a 62-year-old Caucasian man with chronic B-chronic lymphocytic leukemia (B-CLL) in prolymphocytic transformation to B-prolymphocytic leukemia (B-PLL). These cells are considered a serial sister cell line of MEC-1, another well-known and widely used B-CLL cell line.
The MEC-2 cell line was derived before any therapy, which makes it particularly valuable for studying the characteristics and behavior of B-CLL cells in their natural state. This cell line has been extensively utilized in various research studies aimed at understanding the biology of B-CLL and investigating potential therapeutic strategies.
The establishment of the MEC-2 cell line has provided researchers with a renewable and reliable source of B-CLL cells for in vitro experiments. These cells exhibit key features of B-CLL, including abnormal proliferation, resistance to apoptosis, and other characteristics associated with malignant transformation.
MEC1 and MEC2 Cell Lines Derived From B-CLL in Prolymphocytic Transformation
The establishment and characterization of two cell lines, MEC1 and MEC2, that grew spontaneously on two subsequent occasions from the PB of a patient with B-CLL in prolymphocytic transformation. The growth pattern of MEC1 and MEC2 in liquid culture differs markedly. MEC1 cells grow adherent to the vessel wall and give rise only to very tiny clumps while MEC2 cells do not adhere to the plastic wall but form very large clumps. Cytospins stained with MGG show large cells with a plasmacytoid appearance, a basophilic cytoplasm, and one or two prominent nucleoli (Fig. 1). The doubling time of MEC1 is 40 h and of MEC2 31 h.
The phenotypic analysis (Table 1) reveals that both cell lines have the same light (k) and heavy chains (m, d) expressed by fresh parental cells and with the same strong intensity. MEC1 and MEC2 share the expression of mature B cell markers (CD19, CD20, CD21, CD22), but differ in the expression of CD23 and FMC7. MEC1 has a higher percentage of CD23+ cells as compared to MEC2 (89 vs 36%) and is FMC7− (<10%), while MEC2 is FMC7+ (>70%). CD5 has always been negative on MEC1 cells, and it has been lost by MEC2 elements after having been expressed on the surface of a proportion (30%) of cells for several months. All the myeloid markers and the T cell markers tested are negative on both lines. As for the adhesion molecules, both cell lines are CD11a+, CD18+, CD44+, CD49d+, and CD54+ and express at high levels both CD80 and CD86, while the patient's fresh cells were CD80 and CD86 negative (Table 1). CD28 is expressed on a proportion of MEC2 cells (35%), while it is negative on the surface of MEC1 cells.
 Fig. 1 MGG staining of cytocentrifuge smears of MEC1 and MEC2 cells. (Stacchini A, et al., 1999)
Fig. 1 MGG staining of cytocentrifuge smears of MEC1 and MEC2 cells. (Stacchini A, et al., 1999)
Table 1. Major phenotypic features of the cells studied. (Stacchini A, et al., 1999)
| MEC1 (%) | MEC2 (%) | Patient's cells (%) | MEC1 (%) | MEC2 (%) | Patient's cells (%) | MEC1 (%) | MEC2 (%) | Patient's cells (%) | |||
| k | 95 | 95 | 73 | CD23 | 89 | 36 | 11.5 | CD18 | 91 | 99 | 52 |
| λ | 0 | 0 | 0 | CD25 | 9 | 3 | - | CD28 | <1 | 36 | 4 |
| sIgM | 99 | 94 | 91 | CD30 | 83 | 30 | 11.5 | CD29 | 48 | 96 | n.t. |
| sIgD | 97 | 99 | 92 | CD38 | 90 | 99 | n.t. | CD44 | 97 | 87 | 96 |
| HLADR | 99 | 97 | 58 | CD40 | 99 | 98 | n.t. | CD49c | <1 | <1 | 13 |
| CD5 | <1 | <1 | 4.5 | CD45 | 99 | 92 | n.t. | CD49d | 99 | 99 | 76 |
| CD10 | <1 | <1 | <1 | CD95 | 90 | 63 | n.t. | CD54 | 98 | 98 | 3 |
| CD19 | 99 | 99 | 66 | FMC7 | 8 | 72 | 81 | CD56 | 3 | <1 | 2 |
| CD20 | 96 | 96 | n.t. | CD11a | 97 | 99 | 27 | CD80 | 98 | 91 | 5.5 |
| CD21 | 58 | 59 | n.t. | CD11b | 5 | 2 | 30 | CD86 | 98 | 96 | 50 |
| CD22 | 76 | 67 | n.t. | CD11c | 13 | 11 | 27 |
MEC1 and MEC2 Lines Represent Two Stages of Prolymphocytic Leukemia
The EBV-carrying lines MEC1 and MEC2 were established earlier from explants of blood-derived cells of CLL patients at different stages of progression to PLL. Their common clonal origin was proven by the rearrangement of the immunoglobulin genes. The cells were driven to proliferation in vitro by the same indigenous Epstein-Barr virus (EBV) strain. They are phenotypically different and represent subsequent subclones emerging in the CLL population.
All cells in the MEC1 and MEC2 lines express both EBNA-2 and LMP-1. Thus, they correspond to Type III latency (Fig. 2A). The immunoblots detected a higher level of EBNA-2 in the MEC2 cells (Fig. 2B). It is unlikely that the B cell-specific transcription factor ARID3A/Bright which is known to upregulate Cp activity accounts for this difference because the mRNA levels were similar in MEC1 and MEC2. It was about half in comparison to a regular LCL, CBM1-Ral-STO (Fig. 2C). Selected FACS profiles are shown in Fig. 2D. The B cell markers, CD19, CD20 and HLA-ABC, HLA-DR, CD30, CD54/ICAM-1, and CCR7 were detected with similar profiles on both lines, while they differed in the expression of CD38, CD27, CD23, CD21, IL-21R, FMC7, CXCR4, and CXCR10.
Soluble factors produced by activated CD4+ T cells were shown to influence the expression of EBV-encoded proteins and thus change the EBV latency type. Treatment of LCL with IL-21 downregulated EBNA-2 expression thus it changed the latency from Type III to Type IIa. Concomitantly, the cells ceased to proliferate. IL-21 also upregulated LMP-1 protein expression. Similar changes were induced in the MEC lines (Fig. 3A and B). The IL-21-induced plasmacytoid differentiation was substantiated by the expression of Blimp-1 (Fig. 3B). The changes were confirmed by the corresponding promoter activities (Fig. 3C). Wp activity decreased in MEC1 cells and both Wp and Cp activity decreased in the MEC2 cells and the LMP-1 mRNA level was elevated in both lines.
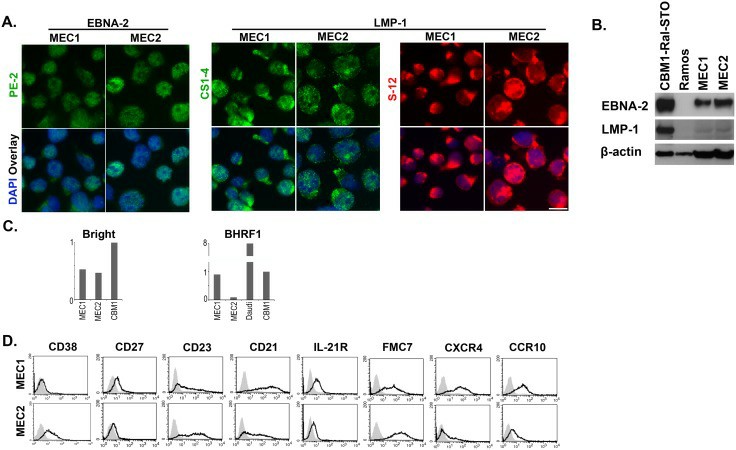 Fig. 2 Comparison of the MEC1 and MEC2 cells. (Rasul E, et al., 2014)
Fig. 2 Comparison of the MEC1 and MEC2 cells. (Rasul E, et al., 2014)
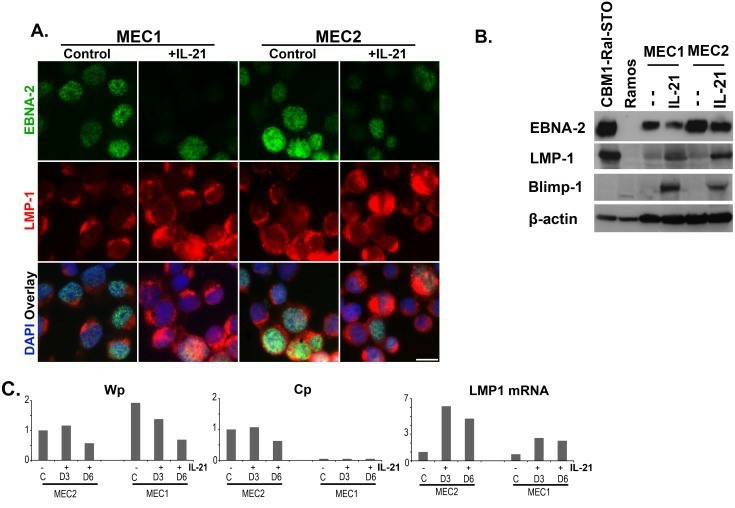 Fig. 3 The effect of IL-21 exposure on MEC1 and MEC2 cells. (Rasul E, et al., 2014)
Fig. 3 The effect of IL-21 exposure on MEC1 and MEC2 cells. (Rasul E, et al., 2014)
It is understood that not all tumors have to be either monoclonal or polyclonal, both types of tumor origin may exist.
MEC-2 is considered a serial sister cell line of MEC-1, another well-known B-cell leukemia cell line.
MEC-2 cells exhibit abnormal proliferation, resistance to apoptosis, and other characteristics associated with malignant transformation, typical of B-cell leukemias.
MEC-2 cells have been extensively used to study the genetic and molecular alterations underlying B-CLL, as well as to investigate the mechanisms involved in the progression of B-CLL to B-PLL. They have aided in understanding the disease biology and identifying potential therapeutic targets.
Ask a Question
Average Rating: 5.0 | 3 Scientist has reviewed this product
New insights
This product has allowed us to study the behavior of cancer cells in a controlled environment, leading to new insights.
12 Jan 2023
Ease of use
After sales services
Value for money
Clear instructions
The clear instructions provided by Creative Bioarray made it easy to prepare and use the medium. I highly recommend this product to researchers working with MEC-2 cells.
13 Oct 2023
Ease of use
After sales services
Value for money
High quality products
The cells arrived promptly, and the packaging was secure, ensuring the quality and viability of the cells.
23 Mar 2024
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need