Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The MT-2 cell line was derived from normal human cord leukocytes of a healthy donor by co-cultivation with leukemic cells from an adult T-cell leukemia (ATL) patient. This unique cell line offers a valuable model for studying certain aspects of T-cell biology and their therapeutic potential.
One significant characteristic of the MT-2 cell line is its ability to produce TGF-β, an immunosuppressive cytokine, which may contribute to the cell line's suppressive activity. Additionally, the MT-2 cell line has been shown to have the phenotypic and functional characteristics of human regulatory T cells (Tregs), suggesting that it may be used as a human Treg-like cell line for studies related to these cells. In addition to its potential role in Treg studies, the MT-2 cell line is also used to detect syncytium-inducing (SI) variants of HIV. In the MT-2 cell culture assay, MT-2 cells are cultivated with cell-free supernatants from HIV-infected PBMC cultures. The inoculated MT-2 cell cultures are then monitored for the development of a typical cytopathic effect, providing valuable insights into the behavior of HIV.
Suppressive Activity of MT-2 Cells on Primary Human PB CD4 Cells and Mouse CD4 Cells
The MT-2 cell line was derived from normal human cord leukocytes of a healthy donor by co-cultivation with leukemic cells from an ATL patient. As shown in Fig. 1a, the proliferative responses of PB CD4 cells to TCR stimulation, as measured by CFSE dilution, were suppressed by 9.7%, 31.6%, 63.3%, and 91.2% after the addition of MT-2 cells at ratios of 10∶1, 10∶2, 10∶5 and 10∶10 (PB CD4 cells/MT-2 cells), respectively. Because the MT-2 cells alone produced high levels of IFN-γ and TNF, but not IL-17A (data not shown), IL-17A was used as an indicator to examine the suppressive effect of MT-2 cells on the cytokine production of cocultured human primary CD4 cells. As shown in Fig. 1b, MT-2 cells potently inhibited IL-17A production over a ratio of 10∶1 to 10∶10 (CD4/MT-2 cells, P<0.05).
Whether the suppressive function of MT-2 cells was allogeneic in manner was investigated. Mouse CD4 cells were used as responder cells to examine the suppressive effect of MT-2 cells. As shown in Fig. 1c, at a ratio of 10∶5 to 10∶10 (CD4/MT-2 cells), MT-2 cells inhibited the proliferation of cocultured mouse CD4 cells by 8% and 51.9%, respectively. However, at a 10∶1 ratio, MT-2 cells stimulated, to a limited extent, the proliferation of cocultured mouse CD4 cells (P>0.05); this was probably caused by an allostimulatory effect of MT-2 cells. Similarly, IFN-γ production by mouse CD4 cells was markedly inhibited by MT-2 cells when cocultured at a ratio of 10∶5 and 10∶10 (CD4/MT-2, P<0.05-0.01), but not at a ratio of 10/1 (P>0.05, Fig. 1d). Although MT-2 cells also suppressed TNF and IL-17 production by mouse CD4 cells (data not shown), they did not suppress IL-2 production (data not shown).
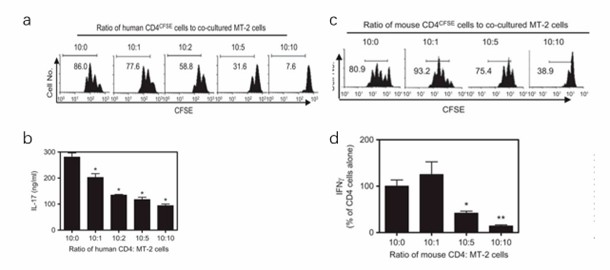 Fig. 1 MT-2 cells inhibit primary human PB CD4 cells and primary mouse CD4 cells. (Hamano R, et al., 2014)
Fig. 1 MT-2 cells inhibit primary human PB CD4 cells and primary mouse CD4 cells. (Hamano R, et al., 2014)
Two Types of HTLV-1 Particles Released from MT-2 Cells
To analyze the distribution and composition of HTLV-I virions, the virus particles were pelleted from MT-2 cell supernatant and were fractionated on sucrose gradients. Fig. 2 demonstrates the distribution of reverse transcriptase (RT) activity across a 30-50% sucrose gradient. As expected, the highest level of RT activity is observed in fractions of density 1.155-1.16 g/cm3. However, a minor peak was detected in fractions of density 1.12-1.13 g/cm3. The amount of [3H]thymidine incorporation in fractions 1.12-1.13 g/cm3 was approximately 5% of that observed in the principal virus-containing fractions. As a control the same reaction mixture was used without exogenous template, yielding a small peak at 1.155 g/cm3.
Fig. 3 shows Western blots of virus-specific proteins in association with fractions of density 1.11 to 1.175 g/cm3 (tracks 1-10). The two monoclonal antibodies recognizing both p19 (MA) and p28 (Fig. 3A and 2B) revealed in fraction 2 (1.12 g/cm3) and fraction 3 (1.125 g/cm3) mostly p28 with less p19 and Pr53gag. However, the level of p28 was lower in fractions from 1.135 to 1.155 g/cm3 (lanes 4, 5, and 6). In the denser fractions (lane 7, 1.165 g/cm3; and lane 8, 1.165 g/cm3) the concentration of p28 increased but was lower than that of p19. To estimate relative amounts of p19 and p28 in the two peak virus-containing fractions, serial twofold dilutions of the virus preparation from fractions 3 and 7 were carried out. The p19 concentration in fraction 7 was nearly eight times higher than that of p28, whereas the amount of p28 in fraction 3 was nearly four times higher than that of p19, indicating that p28 is a major virus protein in the "light" fraction. Using the Mab against p24 (CA) (Fig. 3C), p24 protein was detected mostly in fractions 7 and 8 as expected, with traces in fractions 2 and 3. The low concentration of p24 in fractions 2 and 3 was confirmed with a TSP/HAM patient serum (not shown). Analysis of incorporation of env gene products was carried out by Western blot analysis with an anti-p21E Mab (Fig. 3D). Traces of gp21 (TM) were revealed in fractions 2 and 3 as well as a 32-kDa protein of unknown origin. This protein was also detected in fractions 7 and 8 but in lower amounts than that of gp21 (TM). Since this protein was not previously described among env-coded proteins, it may be of cellular origin. Traces of the SU Env protein, gp46, were also evident in fractions 2 and 3 with the TSP/HAM antiserum (not shown).
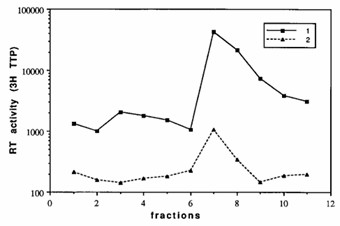 Fig. 2 Reverse transcriptase activity associated with virus particles separated on a sucrose density gradient. (Morozov VA and Weiss RA, 1999)
Fig. 2 Reverse transcriptase activity associated with virus particles separated on a sucrose density gradient. (Morozov VA and Weiss RA, 1999)
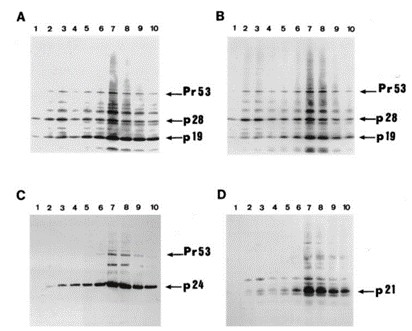 Fig. 3 Comparative analysis of HTLV-1 proteins associated with virus particles of different densities detected by Western blot with monoclonal antibodies. (Morozov VA and Weiss RA, 1999)
Fig. 3 Comparative analysis of HTLV-1 proteins associated with virus particles of different densities detected by Western blot with monoclonal antibodies. (Morozov VA and Weiss RA, 1999)
Plasma cells are white blood cells that produce disease- and infection-fighting antibodies in your body. Myeloma cells prevent the normal production of antibodies, leaving your body's immune system weakened and susceptible to infection.
MT-2 cells have the ability to produce TGF-β, an immunosuppressive cytokine, and they have been shown to have the phenotypic and functional characteristics of human regulatory T cells (Tregs).
Given their phenotypic and functional characteristics of human Tregs, MT-2 cells may be used as a human Treg-like cell line for studies related to these cells.
MT-2 cells, with their unique characteristics and origins, serve as a valuable tool in the study of T-cell biology, Tregs, and HIV.
Ask a Question
Average Rating: 4.7 | 3 Scientist has reviewed this product
Well packaged
Once I received the cells, the product was well packaged and labeled, making it easy to use.
23 Jan 2023
Ease of use
After sales services
Value for money
Good performance
The MT-2 cells have performed well in our experiments, allowing us to make significant progress in our studies.
25 Jan 2024
Ease of use
After sales services
Value for money
Prompt responses
The team at Creative Bioarray was also very helpful, providing prompt and informative responses to our queries.
02 Dec 2023
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need