Featured Products
Our Promise to You
Guaranteed product quality, expert customer support

ONLINE INQUIRY

- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The HuCCA-1 cell line originates from malignant tissue taken from a cholangiocarcinoma patient which developed from biliary epithelial cells. Cholangiocarcinoma stands as the second most common liver cancer following hepatocellular carcinoma (HCC) and presents significant diagnostic challenges and poor patient outcomes especially in areas like Thailand and India. HuCCA-1 is categorized under the intrahepatic form (ICC) of cholangiocarcinoma because it emerges from structures within the intrahepatic bile ducts. HuCCA-1 cells demonstrate epithelial cell characteristics while demonstrating rapid growth inside laboratory cultures. Research demonstrates that these cells establish tumor-like structures which exhibit extensive migratory and invasive capabilities when cultured in 3D environments. These cells demonstrate morphological changes under various conditions which include increased aggregation and cell growth when embedded in collagen I matrices.
The HuCCA-1 cell line is commonly used in anticancer drug assessments and their growth gets inhibited while apoptosis is triggered when exposed to natural substances such as genistein and apigenin. Chemotherapeutics like gemcitabine and cisplatin also exhibit significant inhibitory effects on these cells. Moreover, HuCCA-1 cells in 3D cultures reproduce the in vivo tumor microenvironment which makes them an excellent model to investigate tumor invasiveness and metastasis mechanisms.
 Fig. 1. HuCCA-1 cells(Visitnonthachai D, Nakareangrit W, et al., 2023).
Fig. 1. HuCCA-1 cells(Visitnonthachai D, Nakareangrit W, et al., 2023).
Curcumin Sensitizes CCA Cells to TRAIL-Induced Apoptosis
CCA prognosis is dismal due to late-stage diagnosis, high recurrence, and lack of curative treatment. TNF-related apoptosis-inducing ligand (TRAIL) effectively triggers cancer cell apoptosis yet faces significant resistance in CCA cells due to DcR2 overexpression and anti-apoptotic proteins. Curcumin from turmeric shows cancer cell cytotoxicity by triggering apoptosis through multiple pathways but researchers have not yet examined its effect on TRAIL resistance in CCA cells.
After 24 hours of treatment with curcumin and/or TRAIL, apoptosis was assessed through TUNEL staining. As indicated in Fig. 1A and B, apoptotic cells appeared dark-brown in the nucleus, while non-apoptotic cells appeared light green-brown due to methyl green staining. The results indicated that TRAIL alone did not significantly induce apoptosis in HuCCA-1 and KKU-213A cells, confirming their resistance to TRAIL (Fig. 1C, D). However, 24-hour curcumin treatment prompted apoptosis in both cell lines. The combination of 10 µM curcumin with TRAIL had no effect, but 20 µM curcumin significantly increased apoptosis compared to treatments with curcumin or TRAIL alone. Notably, 20 µM curcumin had a stronger effect on HuCCA-1 cells (10.20 + 2.09-fold of control) than KKU-213A cells (3.23 + 0.05-fold of control). Additionally, 20 µM curcumin combined with TRAIL decreased full-length caspase-8 and increased cleaved caspase-8 in both lines (Fig. 2). This combination also enhanced PARP cleavage, indicating activation of caspase-3, and reduced BID levels, suggesting increased truncated BID (tBID) activity.
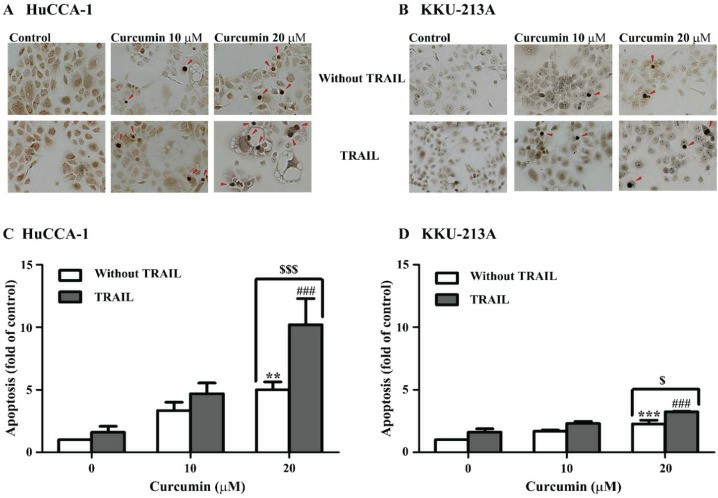 Fig. 1. Curcumin sensitizes cholangiocarcinoma cells to TRAIL-induced apoptosis (Visitnonthachai D, Nakareangrit W, et al., 2023).
Fig. 1. Curcumin sensitizes cholangiocarcinoma cells to TRAIL-induced apoptosis (Visitnonthachai D, Nakareangrit W, et al., 2023).
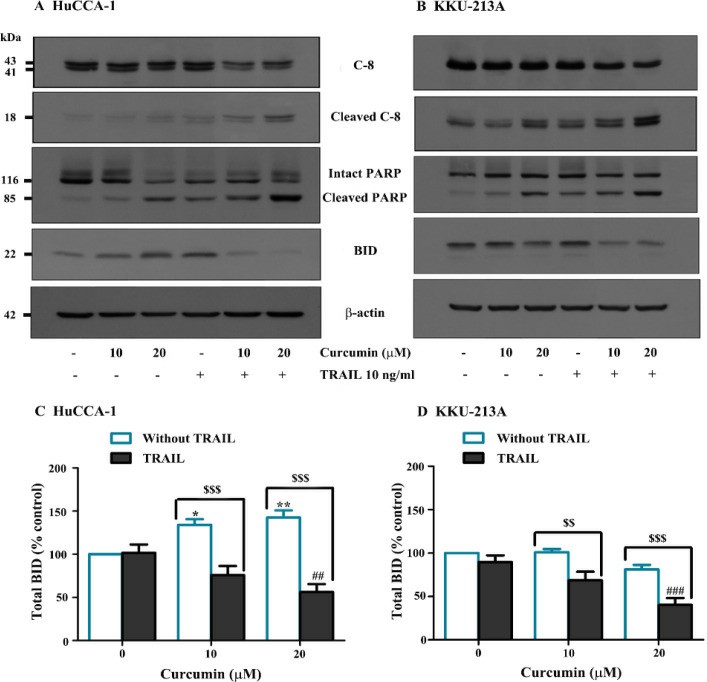 Fig. 2. Curcumin Alters Expression of Proteins Related to Apoptosis (Visitnonthachai D, Nakareangrit W, et al., 2023).
Fig. 2. Curcumin Alters Expression of Proteins Related to Apoptosis (Visitnonthachai D, Nakareangrit W, et al., 2023).
SPHINX31 Attenuated SRSF1 Phosphorylation and Nuclear Localization in Hucca-1 Cells
SRPK1 plays a vital role in mRNA splicing activities and demonstrates high expression levels in multiple cancer types which establishes it as a valuable therapeutic target. Cholangiocarcinoma (CCA) demonstrates low survival rates because it exhibits known overexpression of VEGF-A which leads to abnormal angiogenesis. Supradit et al. investigates SRPK1 expression in cholangiocarcinoma and evaluates how SPHINX31 inhibition affects both cell viability and angiogenesis through VEGF-A splicing modification to decrease pro-angiogenic isoforms.
The cytotoxicity of SPHINX31 (SRPK1 inhibitor) on HuCCA-1 cells was evaluated using the MTT assay. Results showed no significant reduction in cell viability after 24-hour treatment with SPHINX31 at concentrations of 0.3, 1, 3, and 10 μM compared to controls (Fig. 3A). Flow cytometry indicated no significant changes in cell proliferation or apoptosis (Fig. 3B and C). However, RT-qPCR revealed a significant downregulation of VEGF-A165a mRNA to 60% of controls (Fig. 3D). SRPK1 phosphorylates SRSF1, affecting its nuclear localization. Western blot showed reduced SRSF1 phosphorylation at tested SPHINX31 concentrations (Fig. 4A). At 0.3 μM SPHINX31, immunofluorescence indicated diminished SRSF1 nuclear localization (Fig. 4B). These results suggest SPHINX31 suppresses SRSF1 phosphorylation and nuclear localization, decreasing pro-angiogenic VEGF-A165a in HuCCA-1 cells (Fig. 3D).
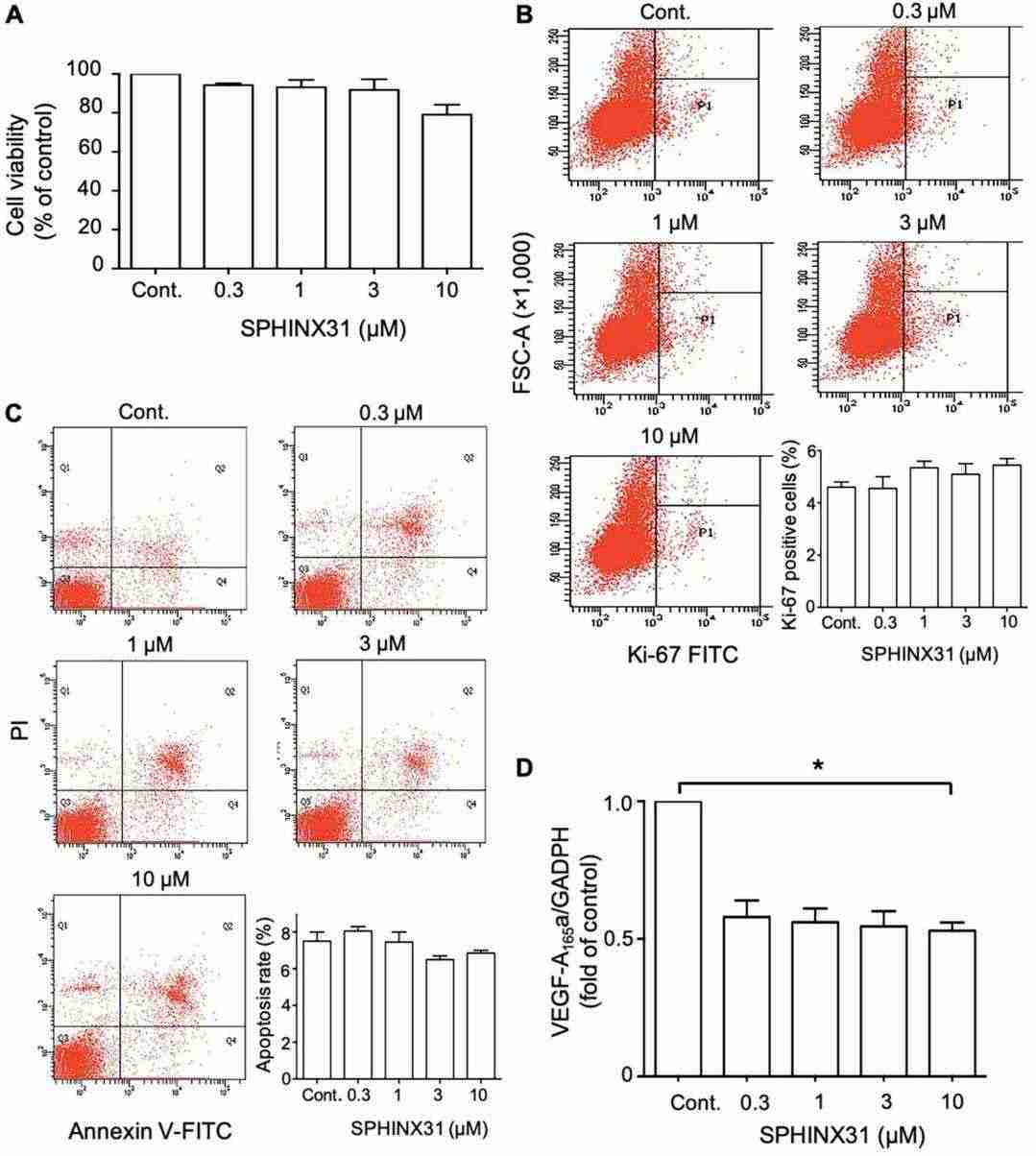 Fig. 3. Effects of SPHINX31 on viability, proliferation, apoptosis and expression of pro-angiogenic VEGF-A165a in HuCCA-1 cells (Supradit K, Boonsri B, et al., 2022).
Fig. 3. Effects of SPHINX31 on viability, proliferation, apoptosis and expression of pro-angiogenic VEGF-A165a in HuCCA-1 cells (Supradit K, Boonsri B, et al., 2022).
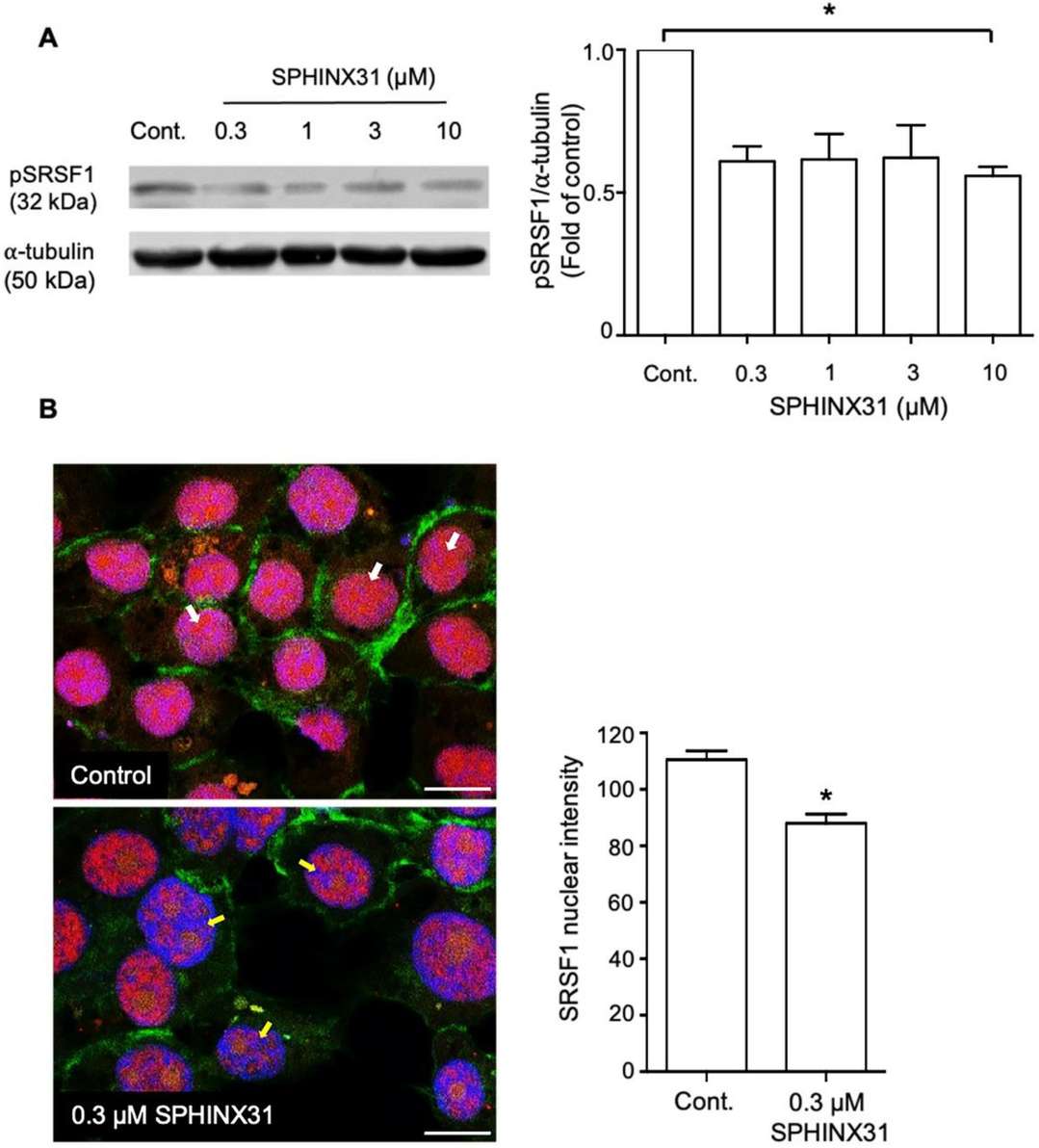 Fig. 4. Effect of SPHINX31 on SRSF1 phosphorylation and nuclear localization in HuCCA-1 cells (Supradit K, Boonsri B, et al., 2022).
Fig. 4. Effect of SPHINX31 on SRSF1 phosphorylation and nuclear localization in HuCCA-1 cells (Supradit K, Boonsri B, et al., 2022).
Most gallbladder cancer begins in the glandular cells that line the inner surface of the gallbladder. Gallbladder cancer that begins in this type of cell is called adenocarcinoma.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Quick delivery
I was impressed with how quickly my cells arrived after placing my order.
16 Nov 2022
Ease of use
After sales services
Value for money
Write your own review
- You May Also Need







