ONLINE INQUIRY

Human Tracheal Fibroblasts
Cat.No.: CSC-8244W
Species: Human
Source: Trachea
Morphology: Fibroblasts
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Never can primary cells be kept at -20 °C.
Human tracheal fibroblasts (HTrFs) support the trachea's integrity and function as a mediator for its injury and repair. The cells mainly generate extracellular matrix (ECM) molecules such as collagen and elastin that support and shape the trachea. In normal circumstances, HTrF activity is tightly controlled by specialized cell signalling networks like the transforming growth factor β1 (TGF-β1) and RHOA systems. These networks control fibroblast proliferation, differentiation, migration, and ECM synthesis and degradation, ensuring the balance of the tracheal environment.
When the trachea is injured by surgery, excessive mechanical ventilation, or harmful gas inhalation, HTrFs become activated. Activated HTrFs proliferate and migrate rapidly, secreting large amounts of ECM at the injury site to aid tissue repair. However, this repair process can be a double-edged sword: while adequate ECM deposition helps restore tracheal structure, excessive HTrFs activation and ECM accumulation can lead to fibrosis. This fibrosis thickens the tracheal wall and narrows the lumen, disrupting normal breathing. Thus, it is essential to understand the cellular properties and regulatory mechanisms of HTrFs in order to shed light on how tracheal diseases arise and how to prevent and treat them. In recent years, novel therapies such as photodynamic therapy and blue light therapy have shown promise in reducing overactive fibroblasts. As research advances, precision medical strategies targeting HTrFs regulation are expected to become increasingly important in the clinical management of tracheal diseases.
POSTN Mediated the Migration and Proliferation of Human Tracheal Fibroblasts through TGF-β1
Laryngeal and tracheal fibroblasts spread and secrete ECM wildly in response to inflammatory and immune mediators. Over-accumulation of ECM creates scars, which in turn causes laryngotracheal stenosis (LTS). Transforming growth factor β1 (TGF-β1) is closely related to cicatricial LTS. Researchers have reported that POSTN, a regulator of the TGF-β pathway, might also be involved, but the molecular mechanisms have not yet been elucidated.
She et al. found that POSTN and TGF-β/RHOA pathway related molecules (TGF-β1, RHOA, CTGF, COL1) were over-expressed at both mRNA and protein levels in LTS tissue. Overexpression or silencing of POSTN in tracheal fibroblasts induced TGF-β activation and cell proliferation and migration via the TGF-β/RHOA pathway. According to the wound healing assay and CCK8 assay, compared with the NC group and empty vector group at the same time, the migration distance and the ability to proliferate in the POSTN-overexpression group was greater; by contrast, the migration distance and the capacity to proliferate in the cells of the POSTN-silenced group were diminished (Fig. 1). The migration distance and proliferation capacity of cells in the overexpression + TGF-β1 inhibitor P144 group were suppressed; the movement distance and proliferation capacity of cells in the silenced + TGF-β1 agonist SRI-01138 group were increased compared with cells in the overexpression + TGF-β1 agonist SRI-01138 group (Fig. 2), suggesting that POSTN induced the migration and proliferation of tracheal fibroblasts via TGF-β1.
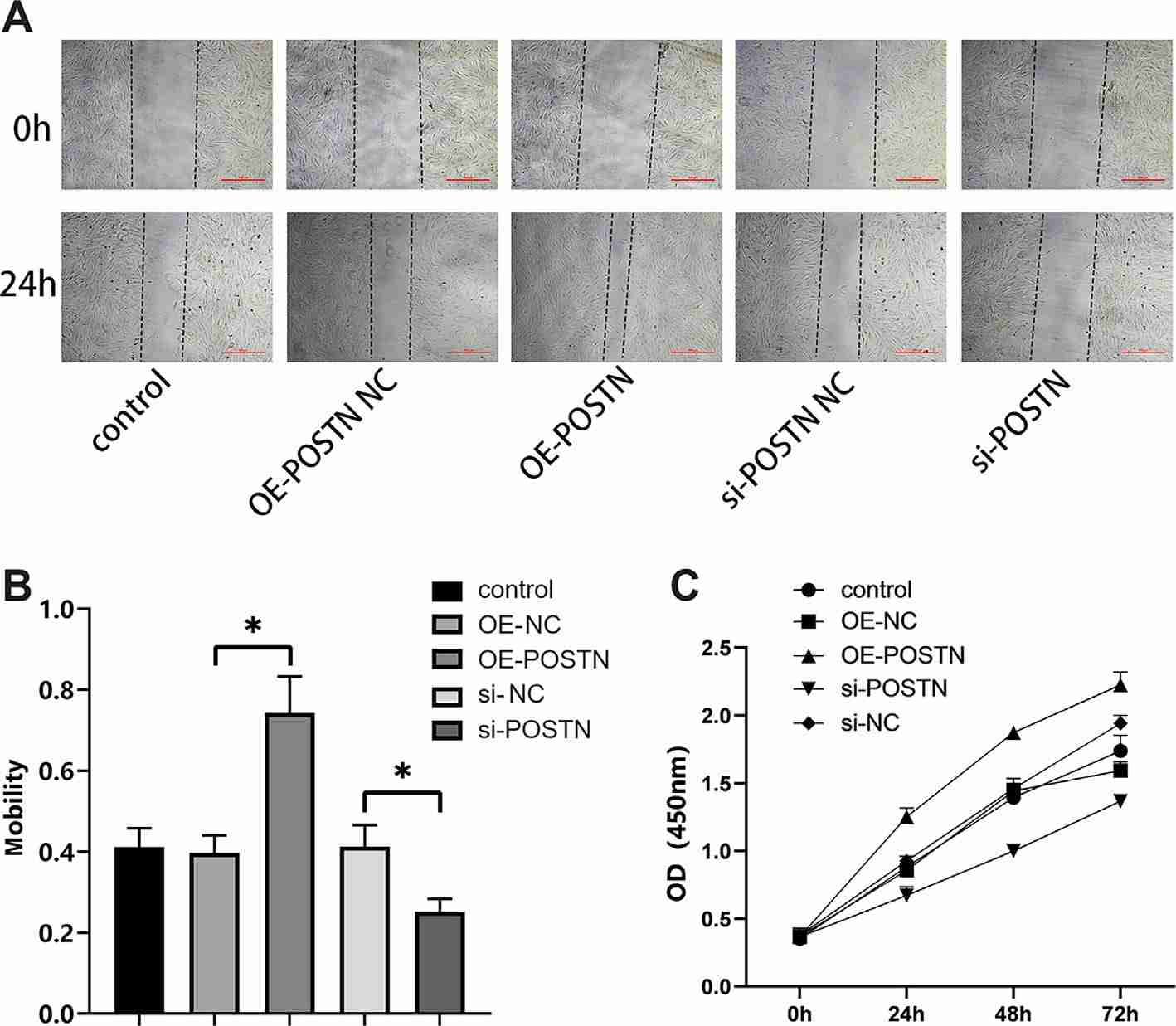 Fig. 1. The effect of periostin (POSTN) on the migration and proliferation abilities of tracheal fibroblasts (She, Z., Chen, H., et al., 2024).
Fig. 1. The effect of periostin (POSTN) on the migration and proliferation abilities of tracheal fibroblasts (She, Z., Chen, H., et al., 2024).
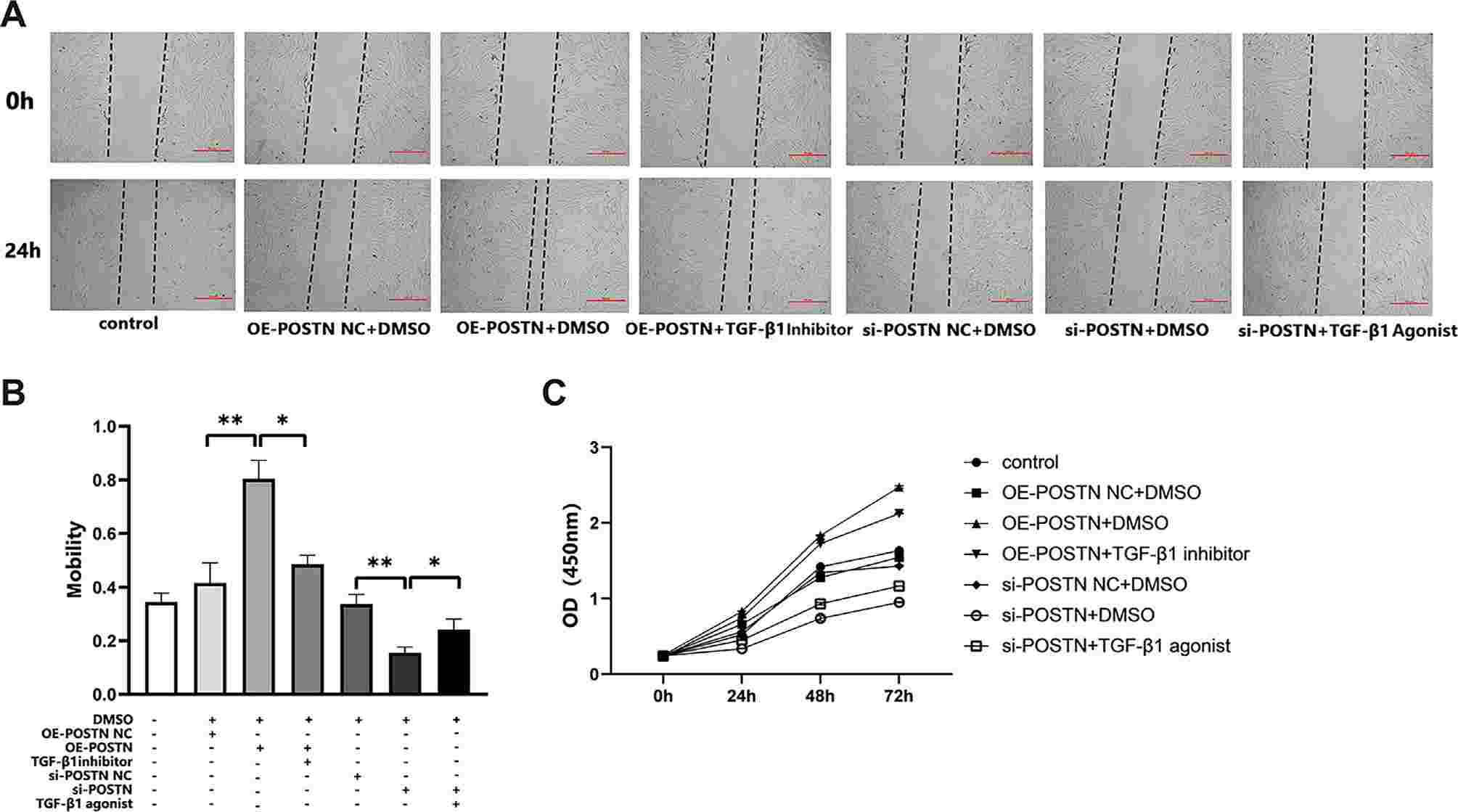 Fig. 2. The effect of periostin (POSTN) on the migration and proliferation of tracheal fibroblasts through transforming growth factor β1 (TGF-β1) (She, Z., Chen, H., et al., 2024).
Fig. 2. The effect of periostin (POSTN) on the migration and proliferation of tracheal fibroblasts through transforming growth factor β1 (TGF-β1) (She, Z., Chen, H., et al., 2024).
Combined Treatment Inhibited TGF-β1-Stimulated Cell Migration and Proliferation, and Regulated the Release of Fibrotic Proteins.
Tracheostomal stenosis is a significant complication following tracheostomy and laryngectomy, often leading to severe clinical problems due to inflammation and fibrosis. Current treatments are insufficient, often resulting in restenosis. Phlorotannins (PT), derived from natural sources, are noted for their anti-fibrotic properties by suppressing TGF-β1-related fibrotic proteins. Photobiomodulation therapy (PBM), especially blue light (BL), has shown potential in reducing fibroblast activity.
Lee's team explored the combined use of phlorotannins (PT) and blue light (BL) therapy as a preventative measure against tracheal stenosis. The cytotoxicity of PT and BL in human tracheal fibroblasts (HTrFs) was assessed using the MTT assay, and the maximal permissible dose of both treatments was quantitatively determined based on the cell viability results. The results showed that, 100 μg/mL PT and 12 J/cm² (200 mW/cm² for 60s) BL were the maximal non-cytotoxic doses and used in further experiments (Fig. 1A-C). The proposed treatment's effects on cell migration and proliferation were tested in TGF-β1-stimulated HTrFs. Images at 0 and 24 hours showed cell boundaries (Fig. 1D). Migration rate, shown as wound closure percentage, increased to 89% ± 6% with TGF (Fig. 1E). PT + BL significantly reduced wound closure to 55% compared to TGF (Fig. 1E). Significant protein release in the TGF group, with type-1 collagen and a-SMA increasing by 122% and 159%, respectively. PT and BL treatments reduced these levels. PT downregulated type-1 collagen and a-SMA by 66% and 87%, respectively; BL reduced them by 60% and 82%. Combined PT + BL treatment further decreased them to 50% and 64% of the TGF group levels (Fig. 1G and H). Hence, PT + BL effectively modulated TGF-β1-induced fibrotic protein release.
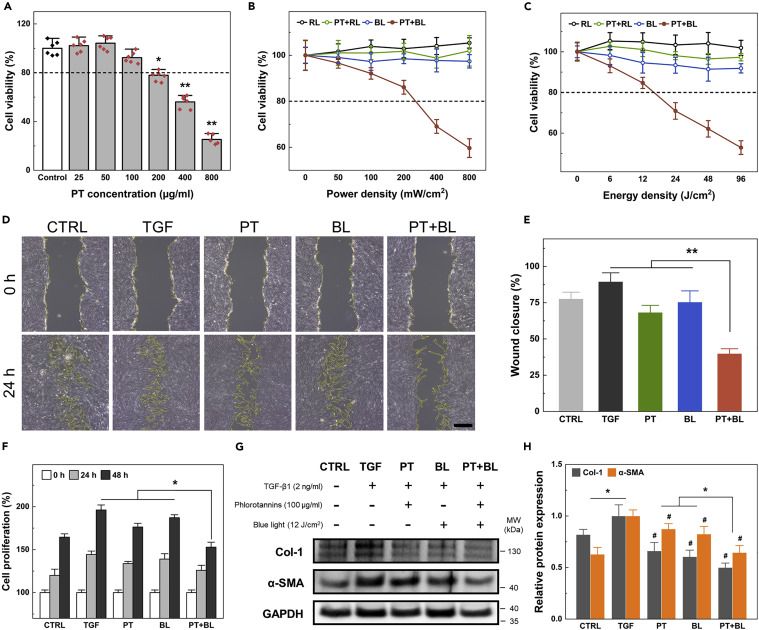 Fig. 1. Combined biomodulative effect of PT and BL on human tracheal fibroblasts (Lee, Y., Heo, S. Y., et al., 2022).
Fig. 1. Combined biomodulative effect of PT and BL on human tracheal fibroblasts (Lee, Y., Heo, S. Y., et al., 2022).
In the trachea, fibroblasts and epithelial cells interact in order to respond to tissue injury, bacterial contamination, and other environmental factors. Studies in vitro and in vivo show that tracheal fibroblasts can promote tracheal epithelial regeneration by influencing epithelial cell proliferation and differentiation. Abnormal proliferation of Human Tracheal Fibroblasts may lead to the development of airway diseases, such as asthma.
Human Tracheal Fibroblasts provided by Creative Bioarray are isolated from human healthy trachea. Human Tracheal Fibroblasts are cryopreserved at passage one and delivered frozen. Each vial of Human Tracheal Fibroblasts contains more than 500.000 viable cells.
Human Tracheal Fibroblasts are a useful model for elucidating the mechanisms of wound healing and may provide novel insights for tissue regeneration.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
Grateful
Our experience at Creative Bioarray has been positive, and we are grateful for the opportunity to advance our research in this area.
22 Sep 2022
Ease of use
After sales services
Value for money
Write your own review