ONLINE INQUIRY
Human Bronchial Fibroblasts (HBF)
Cat.No.: CSC-7822W
Species: Human
Source: Bronchus
Morphology: Fibroblasts
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
HBF are isolated from human bronchus tissue. HBF are cryopreserved at secondary culture (P1) and delivered frozen. Each vial contains >5 x 10^5 cells in 1 ml volume. HBF are characterized by immunofluorescent method with antibody to fibronectin. HBF are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. HBF are guaranteed to further expand for 15 population doublings at the condition provided by Creative Bioarray.
Never can primary cells be kept at -20 °C.
Human bronchial fibroblasts (HBF) and human bronchial epithelial cells participate in an organ-specific communication called the epithelial-mesenchymal trophic unit (EMTU), which is essential for the normal operation of lung tissue. HBF are a primary component of bronchial stromal cells that stem from bronchial tissue. These cells tend to be relatively large and well-rounded, usually as bulging spindle- or stellate-flat structures. Their nuclei are usually oval-shaped and contain large nucleoli.
HBF are very active cells, with a comparatively weakly basic cytoplasm corresponding to strong protein production and secretion. They are able to produce and secrete extracellular matrix elements like collagen and elastin, which are essential for the normal functioning and function of the respiratory tract. Furthermore, HBF also regulate inflammation by reacting to different cytokines and bio-signals through the production of different types of inflammatory mediators. In pulmonary fibrosis, HBF can become proliferative and secrete excessive amounts of extracellular matrix, which can damage and destroy lung tissue. These cells also play a key role in remodeling airways, especially in conditions of respiratory disease like asthma and COPD. When HBF is stimulated and enlarged, the airway wall thickens and fibroses, resulting in respiratory distress and airway blockage. This means that HBF are routinely employed in the scientific literature for the study of lung pathology, drug testing, tissue engineering and immune regulation. They offer crucial experimental platforms and methods for evaluating drugs for pulmonary fibrosis, asthma, COPD and other respiratory diseases.
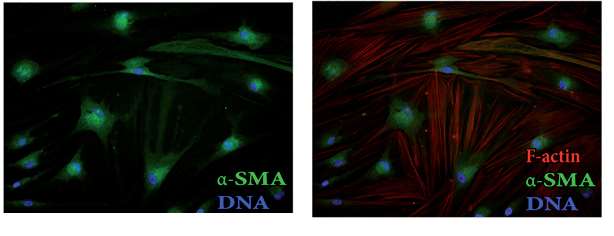 Fig. 1. Images of F-actin cytoskeleton and α-SMA in HBFs (Wójcik-Pszczoła, K., Hińcza, K., et al., 2016).
Fig. 1. Images of F-actin cytoskeleton and α-SMA in HBFs (Wójcik-Pszczoła, K., Hińcza, K., et al., 2016).
BRPM2.5 induces human bronchial fibroblasts differentiation into myofibroblasts and increases the expression of TRPC1.
Household air pollution (HAP) from biomass burning is a significant global health issue, contributing to severe respiratory diseases like COPD. PM2.5, a primary pollutant from HAP, is linked to airway remodeling in COPD but its role in inducing fibrogenic myofibroblast transformation (FMT) remains unclear. Recent studies have found that transient receptor potential classical (TRPC) is involved in mediating cytoskeleton rearrangement of FMT. Therefore, Li et al. investigated whether PM2.5-induced FMT is regulated by TRPC1 function or protein expression and explored the role of the PI3K/AKT signaling pathway in this process, providing insights into fibrosis pathogenesis and potential COPD therapy.
The sensitivity of HBF to BRPM2.5 was assessed using the CCK8 assay, showing no effect on cell viability at concentrations below 30 ug/mL and exposure under 72 hours. However, BRPM2.5 increased a-SMA and COL I expression in a time-dependent manner and caused morphological changes similar to TGF-β1, leading to the differentiation of HBF into contractile myofibroblasts and significant collagen contraction (Fig. 1). These findings suggest BRPM2.5 induces FMT. Then, they examined BRPM2.5's impact on TRPC expression. GPCR analysis showed TRPC1 mRNA levels were higher in HBF than TRPC3, TRPC4, TRPC5, TRPC6, and TRPCZ. HBF exposed to BRPM2.5 increased TRPC1 and TRPC3 mRNA expression but not TRPC4 and TRPC6 (Fig. 2a). They thus focused on TRPC1's role in BRPM2.5-induced FMT. BRPM2.5 treatment elevated TRPC1 mRNA after 12, 24, and 48 hours (Fig. 2b). Western Blot confirmed TRPC1 protein levels rose significantly after 24, 48, and 72 hours of BRPM2.5 exposure (Fig. 2c). Cellular immunofluorescence showed increased TRPC1 protein in HBF after 24 hours of BRPM2.5 stimulation, localized in both the cytoplasm and nucleus (Fig. 2d). These results suggest TRPC1 is involved in BRPM2.5's effects on FMT.
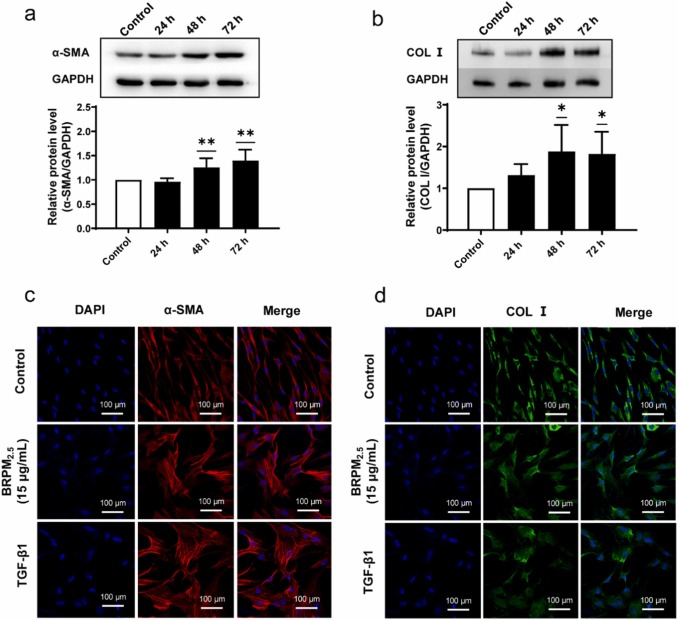 Fig. 2. The effect of BRPM2.5 on fibroblasts (Li, S., Qu, L., et al., 2024).
Fig. 2. The effect of BRPM2.5 on fibroblasts (Li, S., Qu, L., et al., 2024).
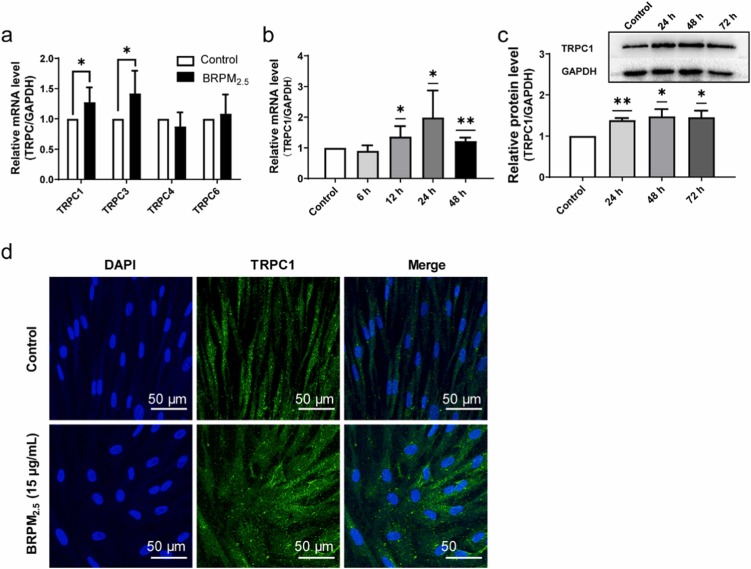 Fig. 2. The effect of BRPM2.5 on TRPC expression in fibroblast-myofibroblast transition (Li, S., Qu, L., et al., 2024).
Fig. 2. The effect of BRPM2.5 on TRPC expression in fibroblast-myofibroblast transition (Li, S., Qu, L., et al., 2024).
IL6 and IL8 Secretion from HBF Treated with Eosinophil-Derived Supernatants Is Dependent on the IL1 Receptor
Eosinophils contribute to allergic inflammation in asthma in part via elaboration of a complex milieu of soluble mediators. Human bronchial fibroblasts (HBF) respond to stimulation by these mediators by acquiring a pro-inflammatory profile including induction of interleukin 6 (IL6) and IL8. Bernau et al. aimed to identifie key eosinophil-derived factors that mediate IL6 and IL8 induction in human bronchial fibroblasts (HBF).
The prior transcriptomic analysis indicated that IL1 signaling might mediate fibroblast responses to eosinophil-derived mediators. To test this, they used IL1RA, an IL1R inhibitor. Treatment with eosinophil supernatants significantly increased IL8 and IL6 secretion by airway fibroblasts, confirmed by ELISA (Fig. 3A and B). Pre-treating HBF with IL1RA reduced IL8 and IL6 protein release, suggesting dependency on IL1 signaling (Fig. 3A and B). They then examined the effect of IL1RA inhibition on HBF-induced CXCL8 and IL6 transcription by eosinophil-derived products. They found that IL1RA reduced the transcription of CXCL8 and IL6 in response to eosinophil-derived products (Fig. 3C and D), indicating the essential role of IL1R in gene upregulation.
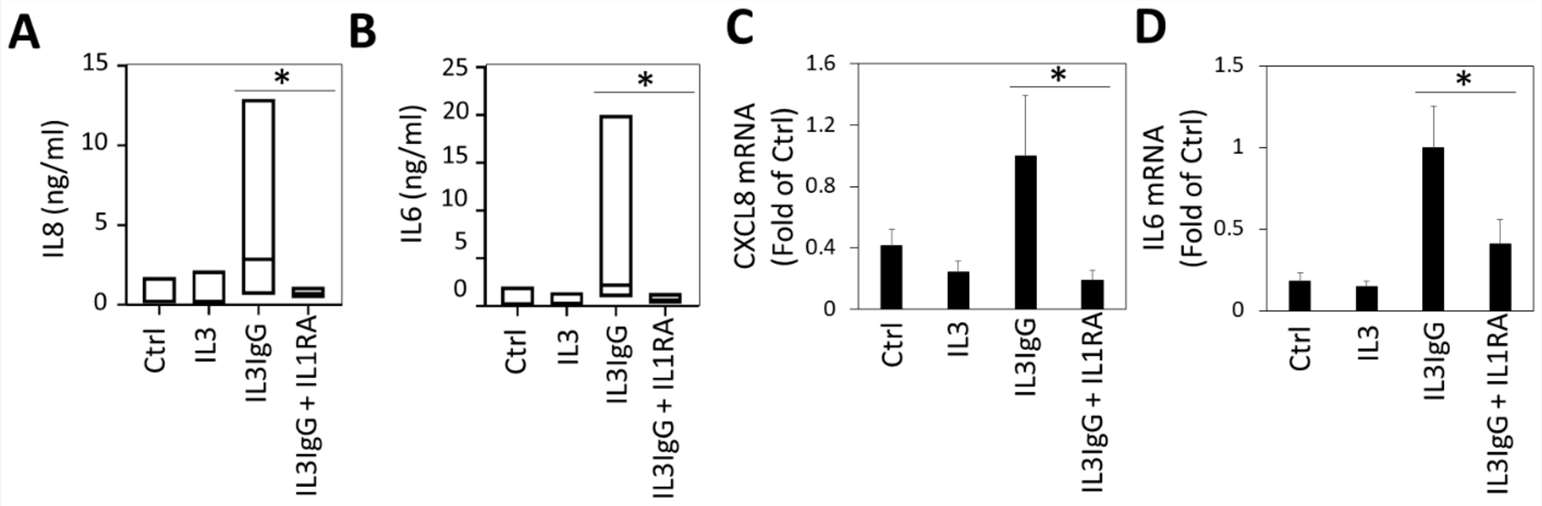 Fig. 3. Interleukin 6 (IL6) and IL8 expression and secretion by human bronchial fibroblasts stimulated with eosinophil-derived soluble mediators requires signaling via the IL1 receptor (Bernau, K., Leet, J. P., et al., 2021).
Fig. 3. Interleukin 6 (IL6) and IL8 expression and secretion by human bronchial fibroblasts stimulated with eosinophil-derived soluble mediators requires signaling via the IL1 receptor (Bernau, K., Leet, J. P., et al., 2021).
It is recommended to use SuperCult® Complete Human Fibroblast Medium (cat# CM-1097X) for the culturing of Human Bronchial Fibroblasts.
Creative Bioarray’s Human Bronchial Fibroblasts provide an ideal culture system to study cell-cell and cell-matrix interactions, airway inflammation, ECM remodeling, fibrosis and wound healing. HBFs from non-diseased donors, as well as donors with well-characterized smoking history, or diseases such as asthma and COPD, are available.
Human Bronchial Fibroblasts (HBF) provided by Creative Bioarray are isolated from human bronchus tissue. HBF are cryopreserved at passage one and delivered frozen. Each vial of HBF contains more than 500.000 viable cells.
Ask a Question
Average Rating: 5.0 | 1 Scientist has reviewed this product
High-quality cell products
Creative Bioarray consistently delivers high-quality cell products that provide key insights into our disease research.
10 Jan 2023
Ease of use
After sales services
Value for money
Write your own review