ONLINE INQUIRY
GFP Expressing Human Hepatic Stellate Cells (GFP-HHSCs)
Cat.No.: CSC-C5459W
Species: Human
Source: Liver
Cell Type: Hepatic Stellate Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Creative Bioarray provides GFP-expressing human hepatic stellate cells (GFP-HHSCs), a cell line that carries green fluorescent protein, to enable GFP-HHSCs to be easily tracked and detected in in vivo and in vitro experiments. HHSCs are unique non-parenchymal cell type in the liver, constitute approximately 8%-13% of the total liver cell population. With their starlike shape and clusters of lipid droplets, HSCs mostly live in the perisinusoidal space (disse's space), between sinusoidal endothelial cells and hepatocytes. HSCs are involved in liver ECM production, which regulates liver fibrosis and microcirculation.
In the physiological state, hepatic stellate cells primarily exist in a quiescent form, functioning as significant vitamin A reservoirs. They regulate ECM homeostasis through autocrine and paracrine production and degradation of several matrix metalloproteinases (MMPs), including MMP-1 and MMP-2. Moreover, HSCs influence microcirculation in hepatic sinusoids with their outward-facing structures, thus modulating hepatic blood flow and portal pressure. Yet when livers become infected with physical, chemical or viral trauma, the body releases cytokines like transforming growth factor-β (TGF-β) and platelet-derived growth factor (PDGF), which transform HSCs into myofibroblasts (MFBs). Activated HSCs exhibit a marked increase in proliferation rates and ECM synthesis capabilities. These cells abundantly express α-smooth muscle actin (α-SMA) and fibrosis-associated markers, secreting ECM that contributes to fibrosis. They also secrete growth factors and chemokines, fostering tumor cell growth and invasion. Consequently, HSC activation represents a central event in hepatic fibrosis, and the pursuit of methods to inhibit HSC activation holds potential clinical significance for the treatment of this condition. GFP-HHSCs can be used to study the distribution, migration and change of these cells, as well as the interaction mechanism between cells, and provide a new perspective for revealing the pathogenesis of liver diseases.
Sirt6 Inhibited HSC Activation by Inhibiting TGF-β Signaling
HSCs, or transdifferentiated hepatic stellate cells, promote liver fibrosis through overproduction of extracellular matrix proteins. One deacetylase called Sirt6 has been associated with many biological processes, including liver fibrosis, but there is no indication that it activates HSCs directly. Zhang's team studied the influence of Sirt6 on HSC activation and liver fibrosis. Through gain- and loss-of-function models, and in conditional Sirt6-knockout mice, they demonstrated that Sirt6 deacetylates Smad2, an important transcription factor involved in transforming growth factor β (TGF-β) signalling, which in turn suppresses fibrogenic gene expression and liver fibrosis.
TGF-β mediates HSC activation and fibrosis via the phosphorylation and nuclear translocation of Smad2/3. Apply Ad-GFP or Ad-Sirt6 to human HSC line LX-2 cells, then add TGF-β to measure the activation of the TGF-β pathway. pSirt6 significantly inhibited TGF-β-induced Smad2 phosphorylation and nuclear translocation (Fig. 1A-C). Through coimmunoprecipitation, Sirt6 sat alongside Smad2 that was activated by TGF-β (Fig. 1D). Deacetylation studies of human embryonic kidney 293 (HEK293) cells revealed that TGF-β and Sirt6 overexpression increased Smad2 acetylation, while Sirt6 overexpression decreased it (Fig. 1D). In a luciferase reporter assay, Sirt6 downregulated Smad2 expression (Fig. 1E). hIP analyses revealed that Sirt6 reduced Smad2 binding to the promoters of the genes Colla1 and Colla2 (Fig. 1F). Thus, Sirt6 deacetylates Smad2, thereby regulating its transcription.
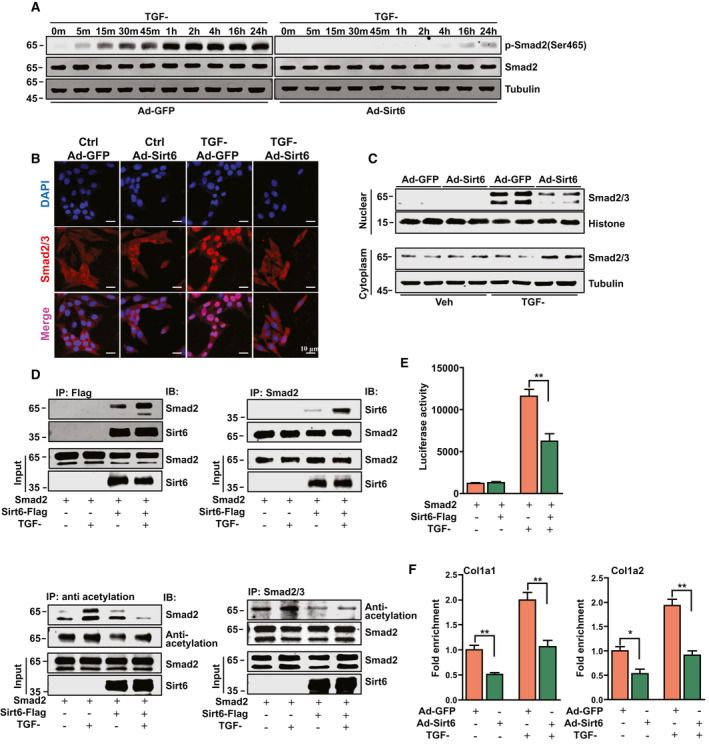 Fig.1. Sirt6 inhibited HSC activation by modulating the TGF-β signaling pathway (Zhang, J., Li, Y., et al., 2021).
Fig.1. Sirt6 inhibited HSC activation by modulating the TGF-β signaling pathway (Zhang, J., Li, Y., et al., 2021).
CXXC5 Over-Expression Suppresses HSC Activation
Myofibroblasts that derive from HSCs largely drive liver fibrosis. The CXXC5 zinc-finger protein, a CXXC-like zinc-finger protein, regulates transcription and has been linked to hepatocellular carcinoma. Wu's team studied the effect of CXXC5 on HSC activation and liver fibrosis.
They showed that CXXC5 overexpression inhibits HSC activation by repressing proto-oncogene MYCL1 transcription, while its inhibition drives HSC activation and fibrogenesis. The human HSC line was infected with an adenovirus containing a FLAG-tagged CXXC5 expression vector (Ad-CXXC5) and an empty vector (Ad-GFP). Over-expression of CXXC5 also reduced expression of several previously well-known pro-fibrogenic genes, such as collagen type I, collagen type III, connective tissue growth factor, and tissue inhibitor of metalloproteinase (Fig. 2A and B). Moreover, CXXC5 overexpression inhibited HSC growth as measured by EdU incorporation (Fig. 2C). Similarly, CXXC5 over-expression reduced pro-fibrogenic gene expression (Fig. 2D and E) and slower growth (Fig. 2F) in primary murine HSCs.
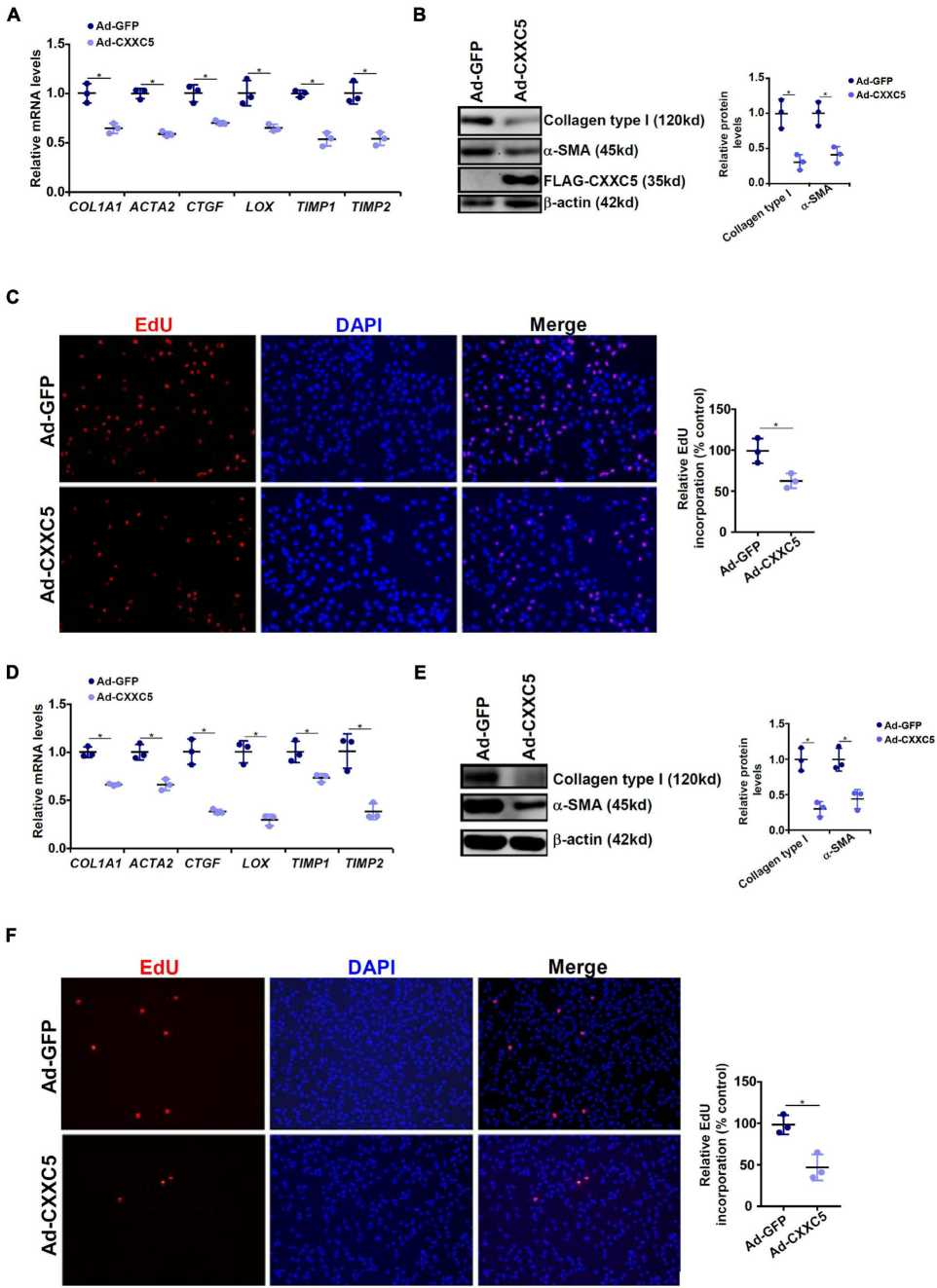 Fig. 2. CXXC5 over-expression suppresses HSC activation (Wu, X., Dong, W., et al., 2021).
Fig. 2. CXXC5 over-expression suppresses HSC activation (Wu, X., Dong, W., et al., 2021).
Ask a Question
Write your own review







