ONLINE INQUIRY
Normal Human Mitomycin-C treated Dermal Fibroblasts
Cat.No.: CSC-C4073X
Species: Human
Source: Dermis; Skin
Cell Type: Fibroblast
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Cell Features:
MCFibs are cryopreserved after secondary culture and treatment with mitomycin C.
MCFibs remain viable and attached in serum-free, animal protein-free, phenol red-free, and antimicrobial-free medium.
MCFibs are extensively tested for quality and optimal performance.
MCFibs are ready to use.
MCFibs are pretreated to help reduce toxic agents in your lab.
Creative Bioarray guarantees performance and quality.
MCFibs are tested for suitability as feeder layers for human embryonic stem cell cultures.
The "Normal Human Mitomycin-C Treated Dermal Fibroblasts" cell line provided by Creative Bioarray is isolated from human skin cells and treated with Mitomycin-C. These dermal fibroblasts form part of a population of mesenchymal cells. They build the cellular signalling microenvironment by emitting a matrix-like ECM and providing both biophysical and biochemical signals. As such, they are crucial to the maintenance of tissue, development and disease. In particular, they are critical for embryonic development, for homeostasis of tissues in adults, for injury response, tissue repair and remodeling.
Mitomycin-C, an antitumor antibiotic, works mostly by blocking the production of DNA. This therapy slows down fibroblast growth, and maintains their normal cellular function and activity. For example, Mitomycin-C is significantly inhibited by fibroblast growth at certain concentrations. When used in dosages of 0.4 mg/mL, it also stimulates the production of two other important cytokines: basic fibroblast growth factor (bFGF) and transforming growth factor-1 (TGF-1). These cytokines simulate the living environment and allow stem cells to grow and develop at their best. Therefore, these Mitomycin-C treated fibroblasts are frequently used as a feeder layer for culturing other cell types, particularly in stem cell cultures where they help maintain the stem cells in an undifferentiated state. Additionally, these Mitomycin-C treated dermal fibroblasts demonstrate unique applicative value in research areas such as gene regulation, exploring skin architecture and function, and investigating the mechanisms underlying related skin diseases.
Nonviral mRNA Delivery via MCMs Improved Transfection in Nonmitotic Primary Human Cells
Nonviral mRNA delivery is an attractive therapeutic gene delivery strategy, as it achieves efficient protein overexpression in vivo and has a desirable safety profile (Fig. 1A). However, mRNA's short cytoplasmic half-life limits its utility to therapeutic applications amenable to repeated dosing or short-term overexpression. Previous studies by Khalil et al. have demonstrated that mineral-coated microparticles (MCMs) enhance transfection efficiency of plasmid DNA and mRNA while reducing cytotoxicity (Fig. 1B). They also stabilize labile growth factors, enabling sustained protein release and prolonged biological response.
Based on these findings, Khalil et al. hypothesized that MCMs could locally deliver mRNA to efficiently produce therapeutic proteins, while sequestering and stabilizing the proteins to extend the duration of the desired biological response. Initially, they used mitotically inactivated primary human dermal fibroblasts (hDFs) to evaluate the effectiveness of MCM-mediated mRNA delivery compared to plasmid DNA (pDNA) delivery. After treatment with mitomycin C to hinder passive pDNA transport, they found that transfection with chemically modified mRNA (CM-mRNA) containing pseudouridine (ψU) and 5-methylcytidine (5-meC) showed higher efficiency than EGFP pDNA (Fig. 1C). CM-mRNA provided a dose-dependent transgene response, demonstrated by increased bioluminescence with CM-mRNA encoding firefly luciferase (Fig. 1D). Transfection with EGFP CM-mRNA lipoplexes from MCMs showed a 1.28-fold increase compared to standard lipofection (Fig. 1E). However, CM-mRNA transfection resulted in short-term expression, with bioluminescence dropping to 21.8% of the peak after 24 hours (Fig. 1D), and MCMs did not extend expression duration.
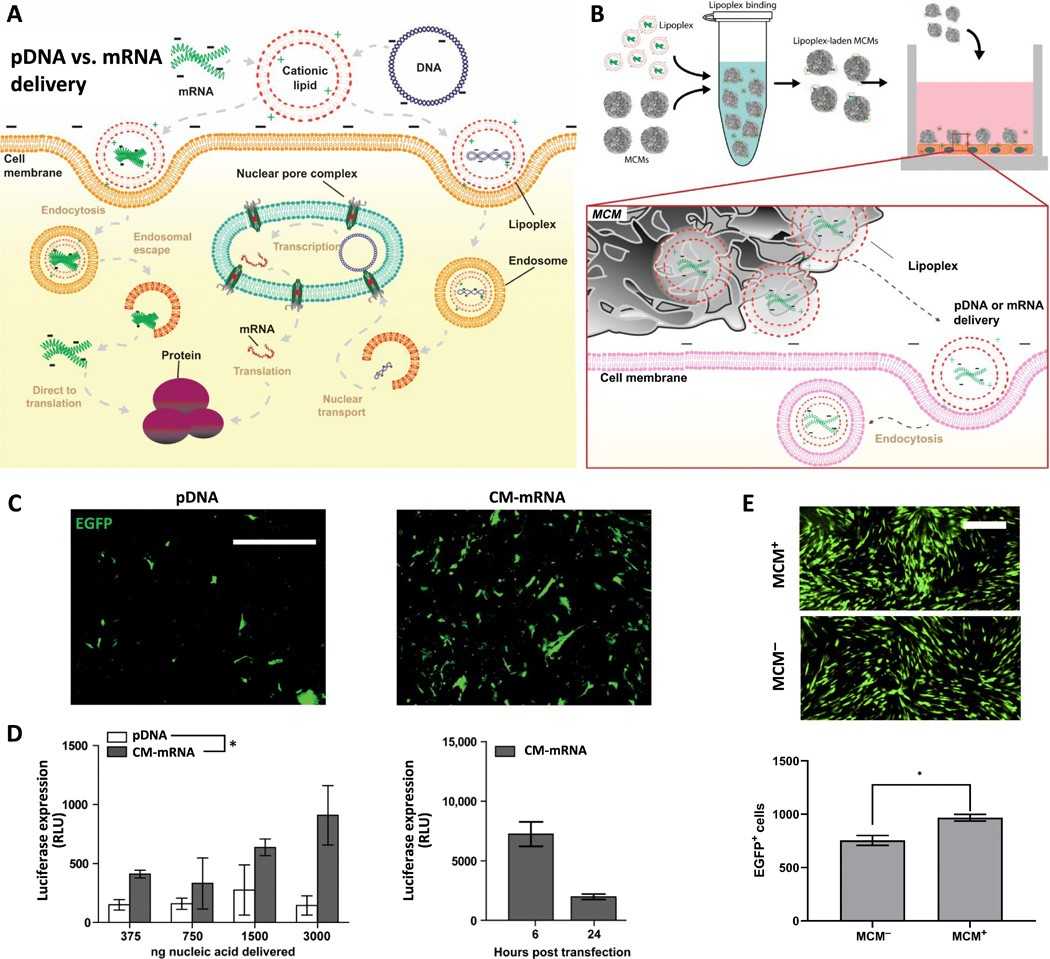 Fig. 1. Mineral-coated microparticle–mediated mRNA delivery is a highly efficient approach for overexpressing transgenes in nonmitotic primary human cells (Khalil AS, Yu X, et al., 2020).
Fig. 1. Mineral-coated microparticle–mediated mRNA delivery is a highly efficient approach for overexpressing transgenes in nonmitotic primary human cells (Khalil AS, Yu X, et al., 2020).
AESIS-1 Increases the Migration Activity of Fibroblasts without Affecting Fibroblast Proliferation
It's common in patients with autoimmune conditions, including rheumatoid arthritis (RA), for wounds to heal slowly. A prompt and successful wound healing is the key to life quality for these patients, so novel skin wound healing materials like bioactive peptides are continually being researched and developed. Previous work on a novel 19-amino acid bioactive peptide, AESIS-1, showed it has therapeutic potential for rheumatoid arthritis. Based on this, Park et al. analyzed the potential of using AESIS-1 as a wound healing drug.
They first tested AESIS-1 peptide's effects on chronic wound healing in a diabetic mouse model, and found that AESIS-1 helped to speed up the mice's wound healing. Rapid cell transfer from the fibroblast to the bed is a key aspect of wound healing. Therefore, they assessed changes in the migration activity of human dermal fibroblast (HDF) cells after treatment with AESIS-1 using a Transwell migration assay. HDF cells were pre-treated with mitomycin C to prevent the proliferative effect. AESIS-1 significantly increased HDF cell migration (Fig. 2a and b), with 20 ng/mL being the most effective concentration, used in subsequent experiments (Figure 2b). After the migration of fibroblasts to the wound areas, it is known that they actively proliferate to heal wounds. Therefore, they tested the effects of AESIS-1 on the proliferation and viability of HDF cells. AESIS-1 did not affect proliferation or viability across a range of treatments (Fig. 2c). Additionally, AESIS-1 did not alter HDF cell morphology (Fig. 2d).
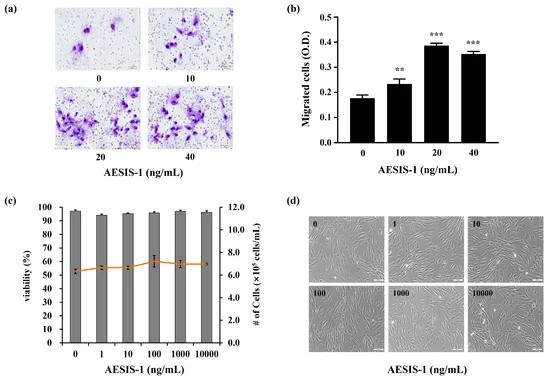 Fig. 2. The AESIS-1 peptide enhances human dermal fibroblast (HDF) cells migration (Park S B, Yang Y, et al., 2024).
Fig. 2. The AESIS-1 peptide enhances human dermal fibroblast (HDF) cells migration (Park S B, Yang Y, et al., 2024).
Ask a Question
Write your own review