ONLINE INQUIRY

Human Epidermal Melanocytes-dark (HEM)
Cat.No.: CSC-7787W
Species: Human
Source: Epidermis; Skin
Cell Type: Melanocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The human epidermal melanocytes-dark (HEM-d) cell line is a highly pigmented, dark melanin-rich melanocyte cell line isolated from human epidermal tissue. These cells exhibit the typical morphology of melanocytes, notably possessing dendritic extensions that facilitate effective communication and interaction with surrounding cells. Primarily located in the basal layer of the epidermis, HEM-d cells engage in close interactions with keratinocytes, transferring melanin granules through these dendritic processes to confer ultraviolet (UV) protection to the skin. In addition, HEM-d cells are integral to regulating cutaneous immune response and antioxidant processes, which are vital for maintaining skin health.
The HEM-d cell line is used in a variety of research applications to study dermatological science, the treatment of skin diseases, and the production and distribution of melanin. Scientists use these cells for in vitro studies designed to explore molecular controls of melanin production and how environmental conditions (UV exposure, chemicals) impact melanin synthesis and distribution. Furthermore, HEM-d cells are used to measure how pharmaceutical agents affect melanocyte proliferation, differentiation, pigment production and survival. The research presents new findings and potential strategies for skin diseases.
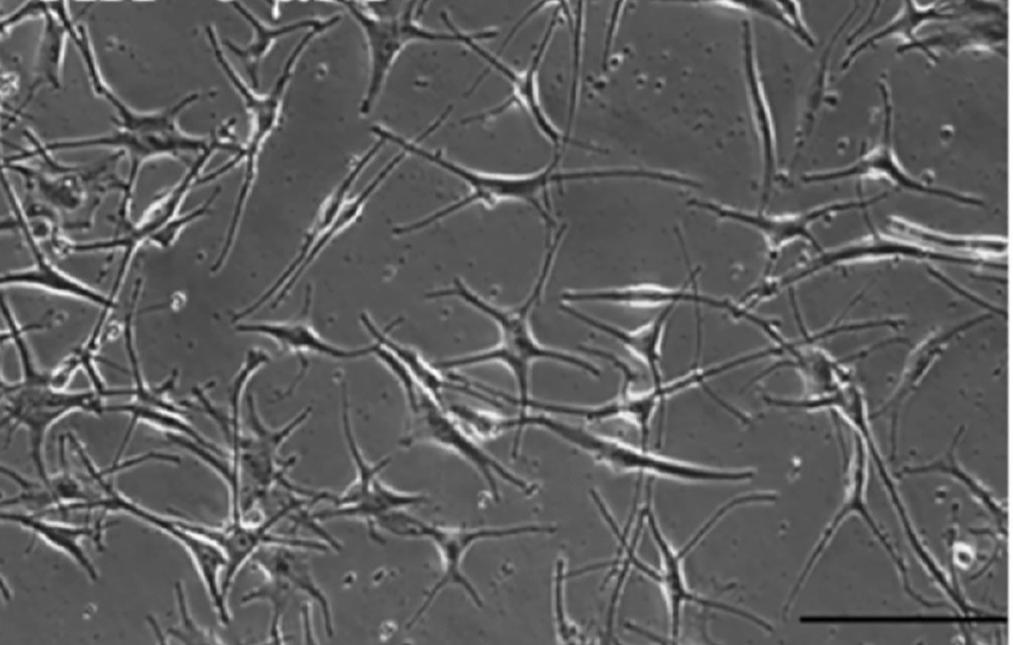 Fig. 1. Human primary epidermal melanocytes from darkly-pigmented (Goenka S, and Simon SR, 2021).
Fig. 1. Human primary epidermal melanocytes from darkly-pigmented (Goenka S, and Simon SR, 2021).
The Influence of Fluoroquinolones and UVA Radiation on Melanin Content in Melanocytes
Fluoroquinolones (FQs) are antibiotics that have a broad spectrum - they're effective against many infections, but also cause phototoxicity, including skin hyperpigmentation, when they attach to melanin. Depending on their chemical structure, FQs vary in phototoxicity, with lomefloxacin and moxifloxacin the most and least phototoxic variants, respectively. Kowalska's team tested the phototoxicity of lomefloxacin and moxifloxacin on melanogenesis in normal human epidermal (dark-pigmented) melanocytes after UVA treatment.
These indicated that 0.05 mM and 0.5 mM omefloxacin decreased melanin by approximately 7% and 14%, respectively (Fig. 1A), and 1.0 mM moxifloxacin reduced melanin by 34 per cent (Fig. 1B). Only UVA exposure heightened melanin by 8 per cent. UVA increased melanin levels (13%-32%) over control when combined with lomefloxacin at all concentrations. In particular, lomefloxacin, both at 0.05 and 0.5 mM, showed greater melanin levels under UVA than cells exposed to untreated irradiation. For moxifloxacin, the 0.01 mM UVA treatment induced an 11% increase in melanin. However, other tested moxifloxacin concentrations did not elevate melanin compared to untreated, irradiated cells (Fig. 1B). Findings reveal that both drugs inhibit melanogenesis, with lomefloxacin enhancing UVA-induced effects differently.
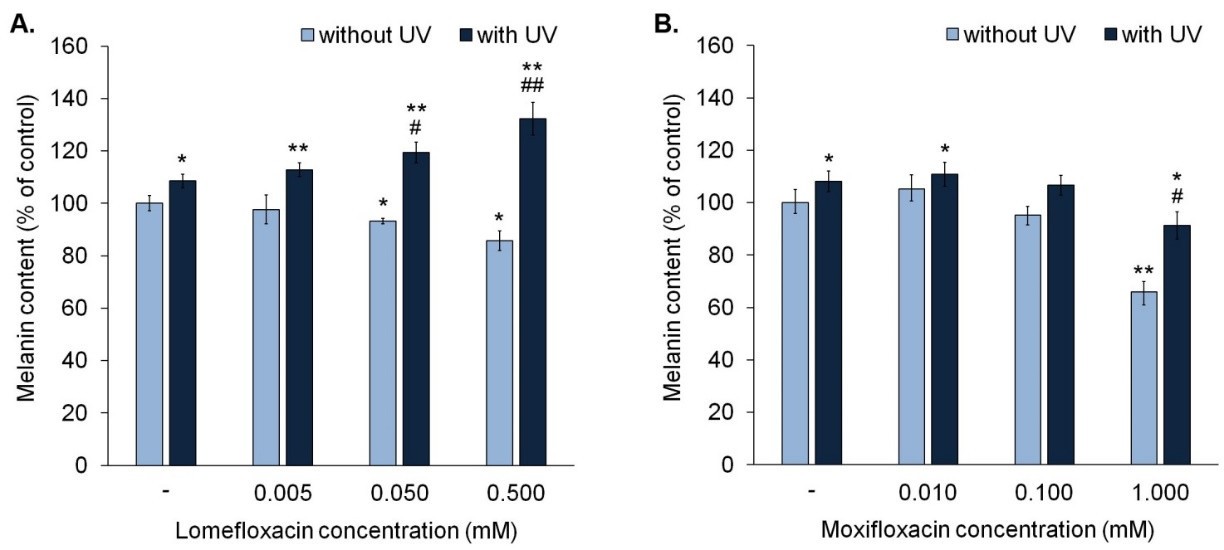 Fig. 1. Fluoroquinolones alone decrease melanin content in melanocytes. Lomefloxacin potentiates the impact of UVA radiation on melanin content (Kowalska J, Banach K, et al., 2021).
Fig. 1. Fluoroquinolones alone decrease melanin content in melanocytes. Lomefloxacin potentiates the impact of UVA radiation on melanin content (Kowalska J, Banach K, et al., 2021).
Effects of Xanthohumol in HEM-DP cells
Xanthohumol (XH) is a hops flavonoid that depigments mouse melanoma cells, but no one knows how it will affect human melanocytes. Flavonoids were shown to modulate the production and transport of human melanin – it's important to find out how XH could be used in human melanocyte cells.
In order to determine if XH is anti-melanogenic in normal melanocytes, Goenka et al. assessed cell death, melanin production, and tyrosinase activity following XH treatment in darkly-pigmented (HEM) human primary epidermal melanocytes. They discovered that XH was cytotoxic at 10 M, which cut down viability by 65.97%; concentrations less than 10 M were non-toxic and were used as control. XH inhibited tyrosinase activity by 36.57% at 2.5 μM and 47.61% at 5 μM (Fig. 2B) but did not affect melanin synthesis (Fig. 2C). As XH did not affect melanin biosynthesis in HEM-DP cells, they then tested whether XH could suppress melanosome export (by measuring the density of the cell). XH changed the cell structure at 2.5 and 5 M, resulting in fewer branched dendrites than the control (Fig. 3A). The number of dendrites decreased by 25.22 and 27.78%, respectively (Fig. 3B). Longitudinal dendrite length dropped by 15.09% and 20.5% at 2.5 M, respectively (Fig. 3C). Multi-dendritic cell counts dropped 39% and 48%, respectively, at 2.5 and 5 M (Fig. 3D). Taken together, the results demonstrate that XH inhibits melanosome export without affecting melanin synthesis.
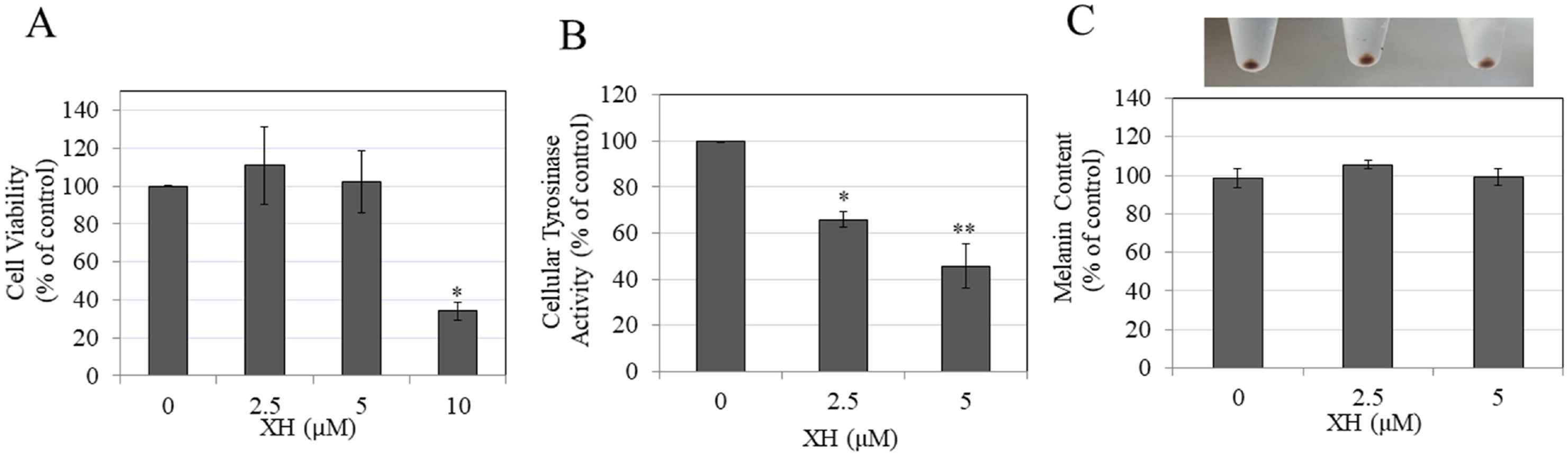 Fig. 2. Viability, melanin synthesis and cellular tyrosinase activity of HEM-DP cells in the presence of different concentrations of XH (Goenka S, and Simon SR, 2021).
Fig. 2. Viability, melanin synthesis and cellular tyrosinase activity of HEM-DP cells in the presence of different concentrations of XH (Goenka S, and Simon SR, 2021).
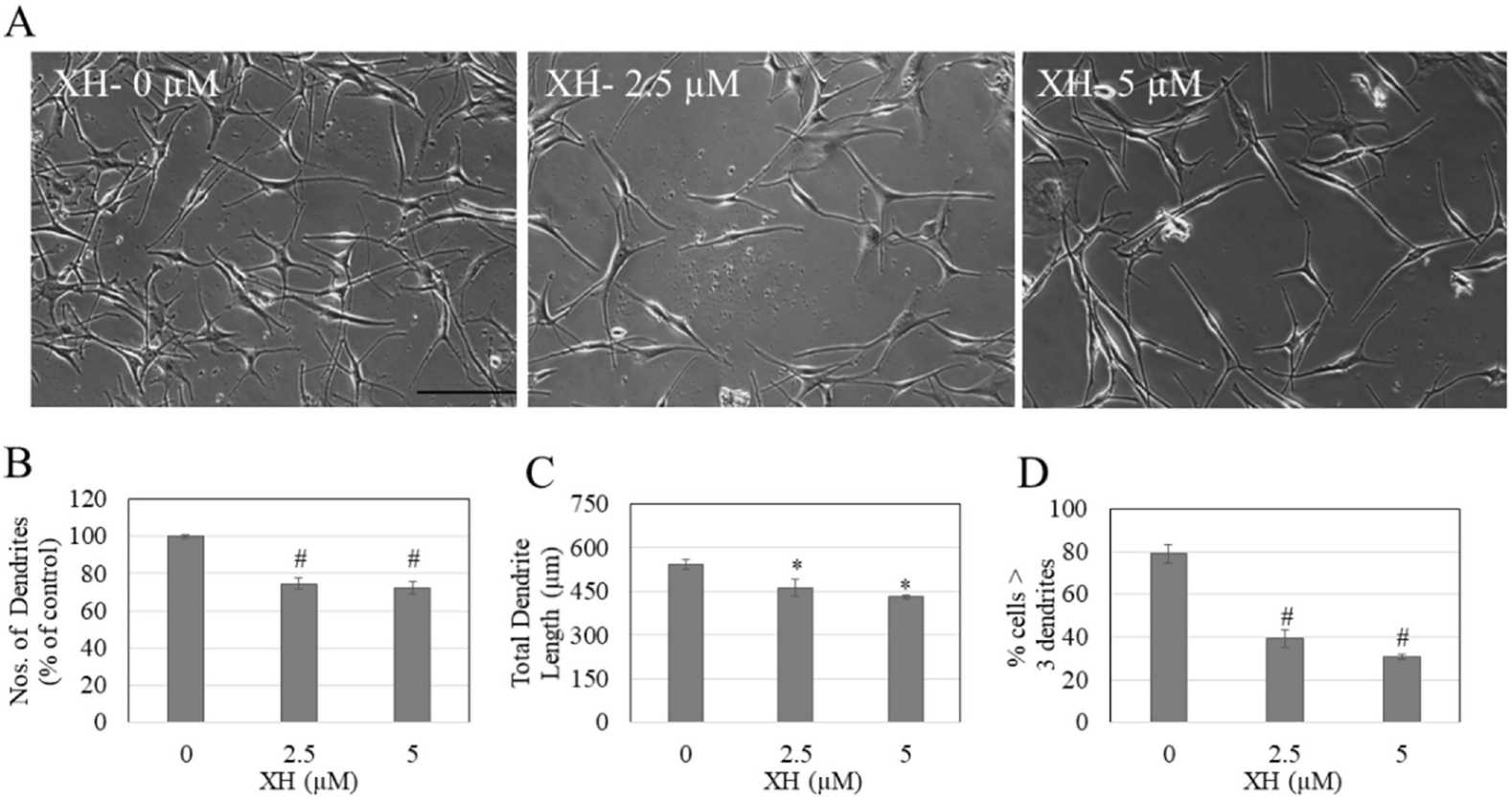 Fig. 3. HEM-DP cell morphology and dendricity measurement (Goenka S, and Simon SR, 2021).
Fig. 3. HEM-DP cell morphology and dendricity measurement (Goenka S, and Simon SR, 2021).
Ask a Question
Write your own review