ONLINE INQUIRY

Human Epidermal Melanocyte-adult (HEM-a)
Cat.No.: CSC-7788W
Species: Human
Source: Epidermis; Skin
Cell Type: Melanocyte
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Human epidermal melanocytes-adult (HEM-a) are derived from the skin tissue of adults. They are prevalent throughout the epidermal layer of the body, notably in regions such as the skin, eyes, inner ear, and leptomeninges. However, due to their inability to autonomously migrate to these organ sites, this characteristic may explain certain congenital conditions such as piebaldism and heterochromia of the iris. A pivotal function of HEM-a is the synthesis and transport of melanin to adjacent keratinocytes, thereby playing a substantial role in shielding the skin from ultraviolet (UV) damage. Additionally, these melanocytes are involved in the immune response, antioxidative activities, and repair processes of the skin. In response to external stimuli like UV exposure or inflammatory agents, they release a variety of bioactive substances that regulate the skin's immune status and mitigate potential free radical damage, thus maintaining skin health and integrity.
In the realm of scientific research, HEM-a are extensively utilized for exploring how melanocytes in adult skin respond to environmental changes. This includes studying the effects of factors such as UV irradiation and chemical exposure on these cells. Moreover, these cell lines are employed to investigate the pathomechanisms of skin diseases, monitor the healing process post-skin injury, and aid in the development of skincare and therapeutic products tailored for adult skin. By assessing the impact of drugs and therapeutic interventions on melanocytes, HEM-a offer novel insights for personalized treatments of skin disorders, fostering innovation in the fields of dermatology and wellness.
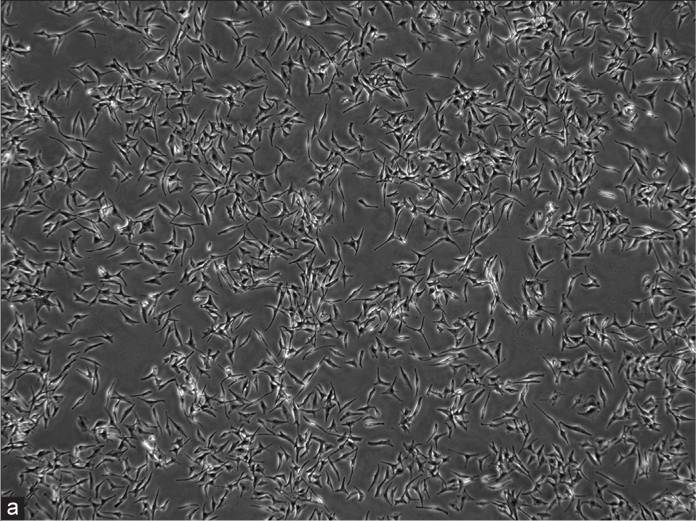 Fig. 1. Human epidermal melanocyte-adult (Shi HX, Zhang RZ, et al., 2022).
Fig. 1. Human epidermal melanocyte-adult (Shi HX, Zhang RZ, et al., 2022).
CCN1 Stimulates Melanogensis in NHEMs through Integrin α6β1, p38 MAPK, and ERK1/2 Signaling Pathways
Fibroblast-derived paracrine mediators like CCN1 (Cyr61) play a vital role in regulating melanogenesis. CCN1, a multifunctional protein, interacts with cell surfaces via integrins to mediate several biological processes. Previously, CCN1 has been linked to diseases such as psoriasis, photoaging, and is activated by UV exposure. However, its role in melanocyte function has not been fully explored.
Therefore, Xu's team aimed to investigate how CCN1 influences melanogenesis. By measuring melanin levels, tyrosinase activity, and the levels of melanogenic factors including MITF, TRP-1, and tyrosinase in CCN1-treated NHEMs, they were able to show that CCN1 regulates melanogenesis through upregulation of these critical factors. To discover the precise molecular mechanisms of CCN1-induced melanogenesis, the integrin profile for CCN1 was detected by measuring the mRNA expression of integrins and in CCN1-injected NHEMs. They found that the CCN1-treated NHEMs showed increased mRNA and protein levels of integrin 6 and 1 subunits (Fig. 1a-d). Specialised siRNA knockdown of these integrins reversed the melanin production and activity of melanogenic enzymes such as MITF, TRP-1, and tyrosinase (Fig. 1e-i). Treatment with CCN1 triggered the p38 MAPK and ERK1/2 signaling cascades, with phosphorylation peaking 5 minutes and 12 hours later, respectively (Fig. 2a-d). The loss of integrins 6 and 1 blocked these pathways (Fig. 2e), indicating their regulatory role. Blockers of p38 MAPK and ERK1/2 remediated CCN1 effects (Fig. 2f-j). All together, these findings indicate that CCN1-induced melanogenesis of NHEMs is mediated through activation of p38 MAPK and ERK1/2.
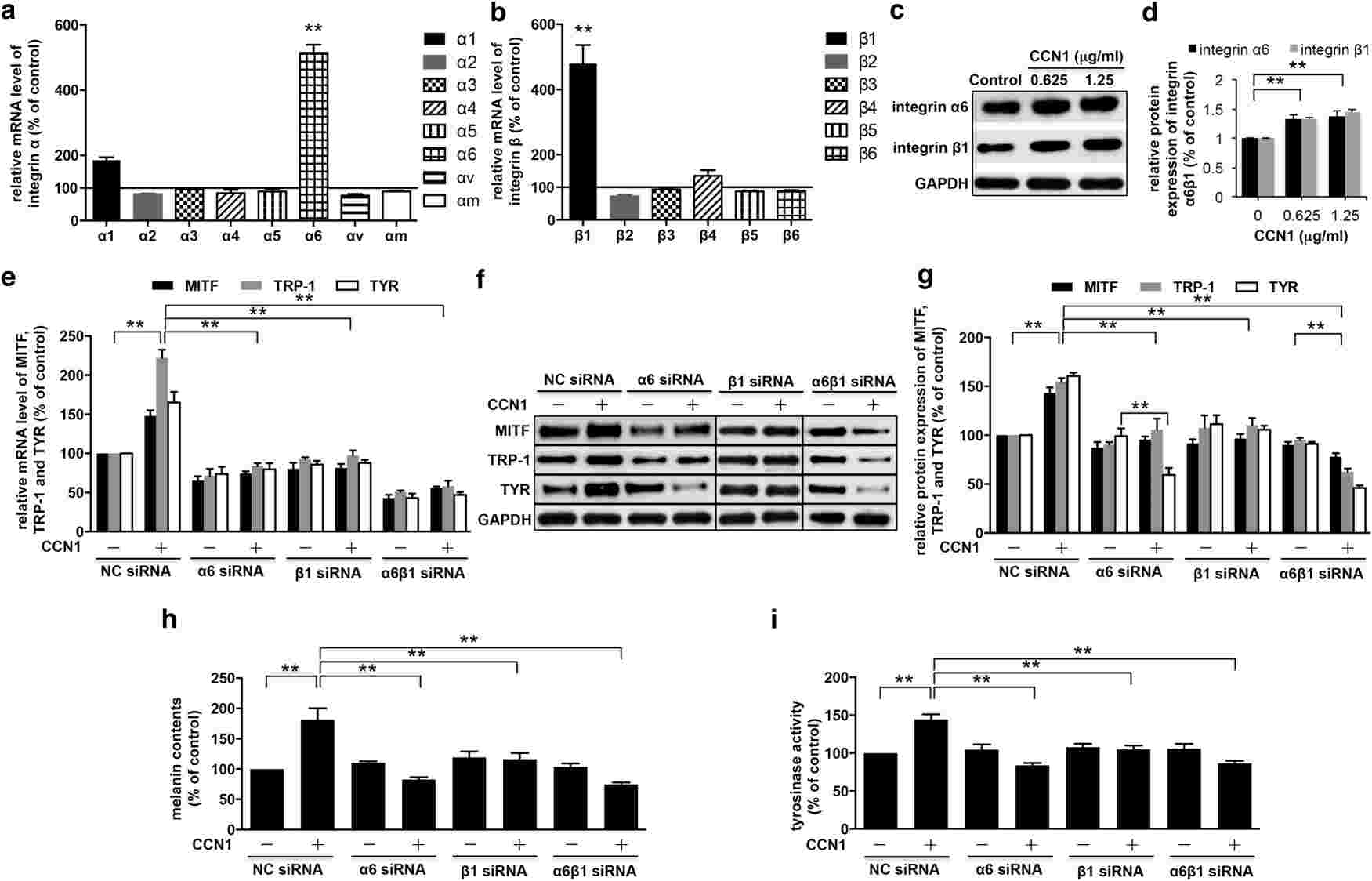 Fig. 1. CCN1 induces melanogenesis via the activation of integrin α6β1 on NHEMs (Xu Z, Chen L, et al., 2018).
Fig. 1. CCN1 induces melanogenesis via the activation of integrin α6β1 on NHEMs (Xu Z, Chen L, et al., 2018).
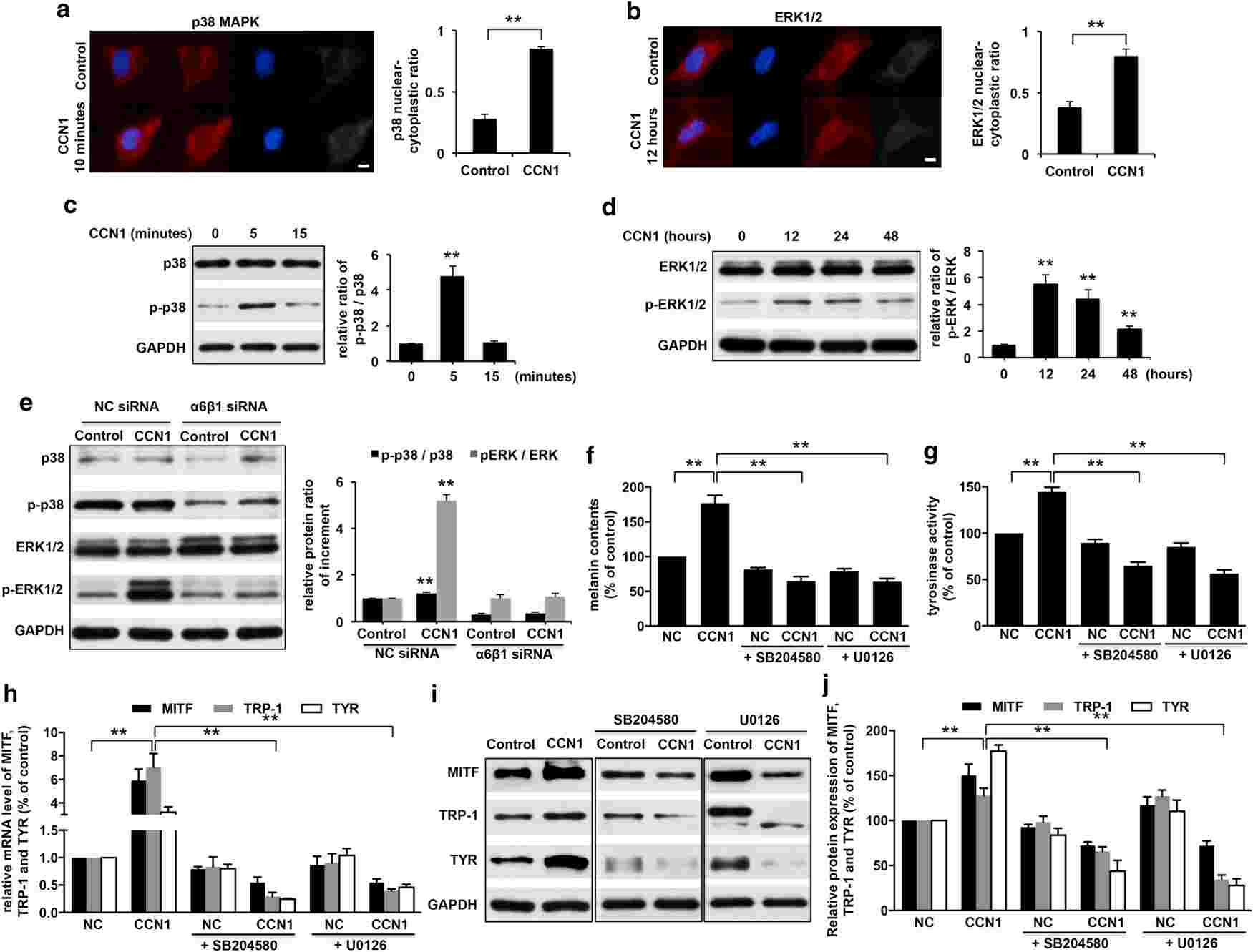 Fig. 2. CCN1 induces melanogenesis via the activation of p38 MAPK and ERK1/2 signaling pathways in NHEMs (Xu Z, Chen L, et al., 2018).
Fig. 2. CCN1 induces melanogenesis via the activation of p38 MAPK and ERK1/2 signaling pathways in NHEMs (Xu Z, Chen L, et al., 2018).
Effects of Keratinocyte-Derived and Fibroblast-Derived Exosomes on Human Epidermal Melanocytes
Exosomes are endosomal extracellular vesicles that can contain proteins, mRNA, microRNA and cytokines. Those molecules can be exported to other cells, and regulate the activity of these receptor cells. Shi's team investigated the activity of exosomes made by keratinocytes and fibroblasts in human epidermal melanocytes.
They divided melanocytes into three groups: blank (melanocytes), Group 1 (melanocytes with fibroblast-derived exosomes) and Group 2 (melanocytes with keratinocyte-derived exosomes). The exosomes were labeled with PKH67 dye. Bright green particles were seen in Group 2 melanocytes, located in the cell bodies and dendrites (Fig. 3a and b), but not in Group 1. After 48 hours, no significant difference was noted in cell density between the blank group and Group 1(Fig. 3c), but Group 2 melanocytes proliferated rapidly and were densely packed (Fig. 3c). All groups showed typical melanocyte morphology. The blank group and Group 1 had similar bipolar or tripolar dendrites (Fig. 3c). In contrast, Group 2 showed multiple dendrites clustering together, unlike the others (Fig. 3c). Tyrosinase activity was measured, showing that keratinocyte exosomes significantly increased activity compared to the blank group and Group 1. No difference was found between Group 1 and the blank group (Fig. 4a). Analyses of melanin content showed that keratinocyte exosomes significantly increased melanin compared with both the blank and Group 1 groups (although no difference was observed between Group 1 and the blank group) (Fig. 4b). Together, keratinocyte-derived exosomes were digested by melanocytes that co-cultured with them and facilitated their growth, tyrosinase activity and melanin production. Yet fibroblast exosomes did not act the same way on melanocytes.
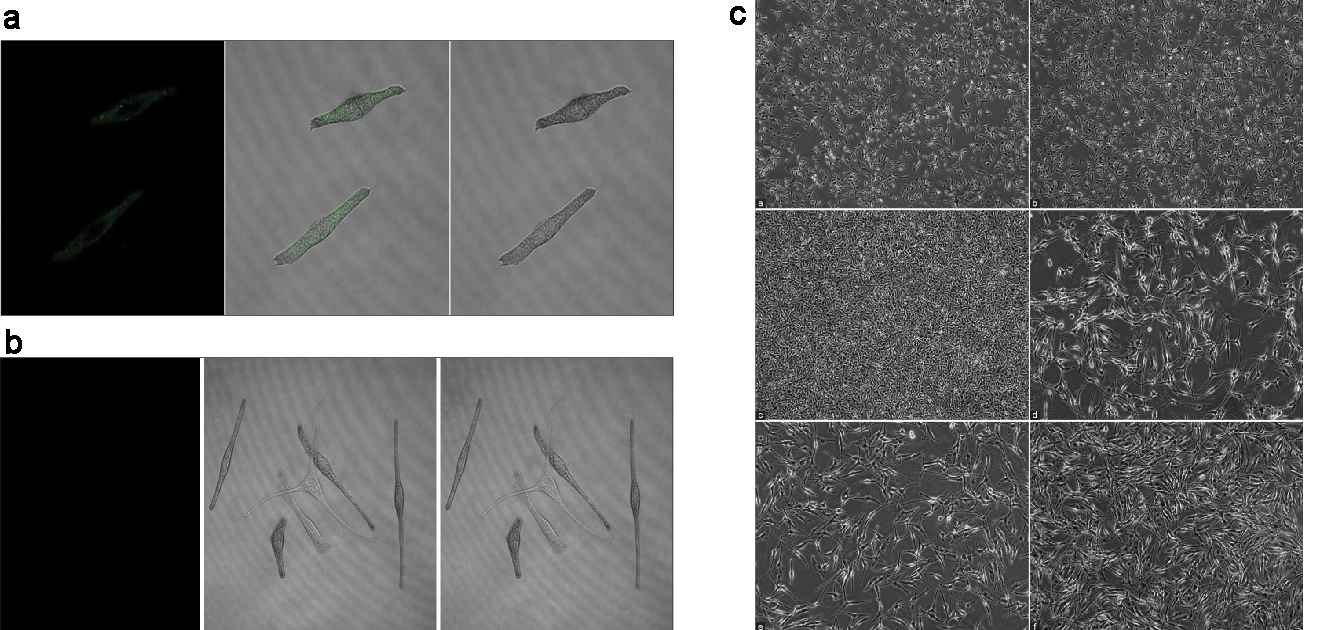 Fig. 3. (a-b) Characteristics of the internalization of exosomes in melanocytes. (c) Morphological observations of melanocytes (Shi HX, Zhang RZ, et al., 2022).
Fig. 3. (a-b) Characteristics of the internalization of exosomes in melanocytes. (c) Morphological observations of melanocytes (Shi HX, Zhang RZ, et al., 2022).
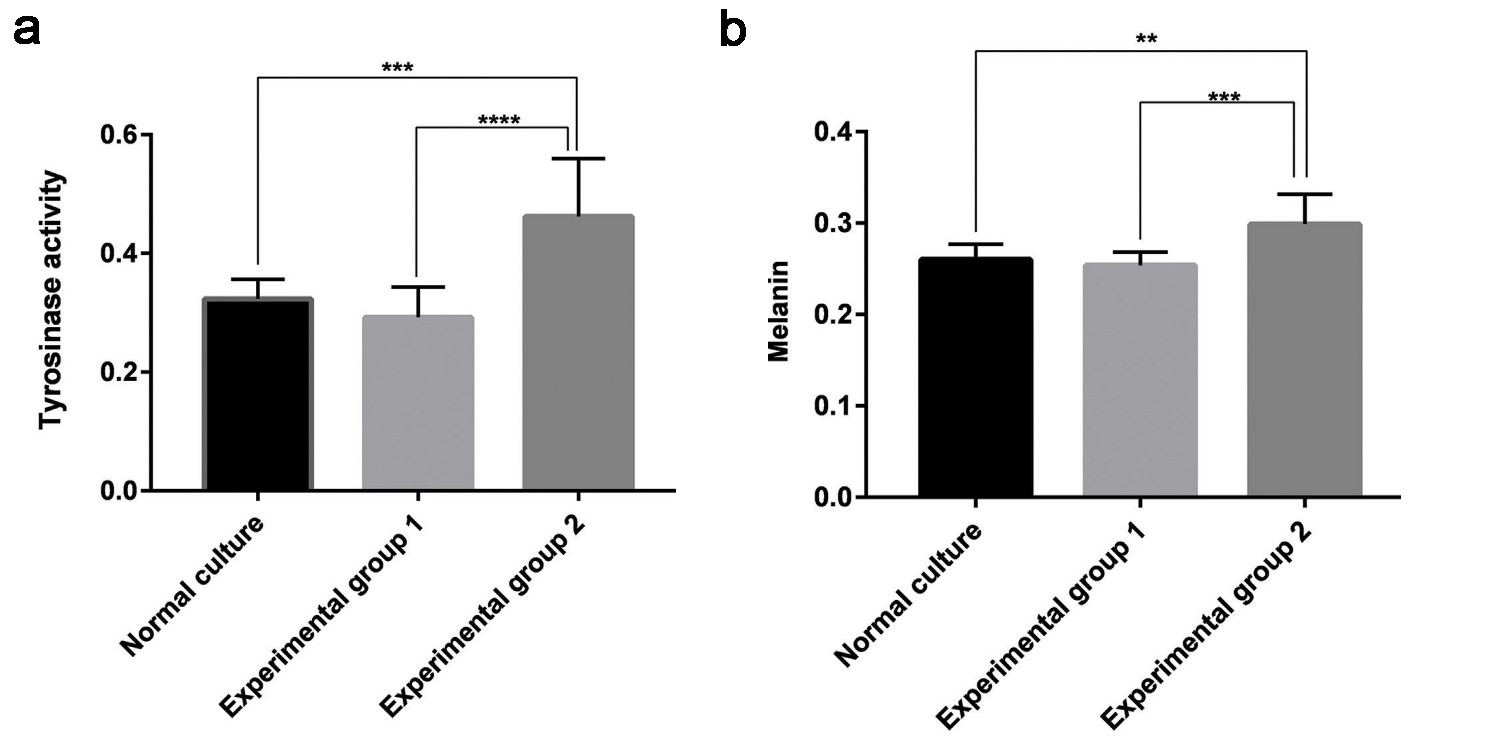 Fig. 4. (a) Tyrosinase activity of the three groups of melanocytes. (b) Melanin content of the three groups of melanocytes (Shi HX, Zhang RZ, et al., 2022).
Fig. 4. (a) Tyrosinase activity of the three groups of melanocytes. (b) Melanin content of the three groups of melanocytes (Shi HX, Zhang RZ, et al., 2022).
Ask a Question
Write your own review