ONLINE INQUIRY

Human Monocyte-Derived Dendritic Cells
Cat.No.: CSC-C4086X
Species: Human
Cell Type: Dendritic Cell
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
Creative Bioarray's Dendritic Cells are derived from monocytes that have been cultured with IL-4 and GM-CSF. Our Dendritic Cells are quality tested via flow cytometry to ensure proper expression of dendritic markers. Dendritic cells express CD11c and HLA-DR. The expression of CD3 (T cells), CD19 (B cells), CD14 (monocytes/macrophages), and CD80 (a maturation marker) is also analyzed to ensure depletion of these cell types.
Cell Features:
Dendritic Cells are derived from monocytes cultured with interleukin 4 and granulocyte-monocyte colony stimulating factor.
Dendritic Cells are extensively tested for quality and optimal performance.
Creative Bioarray guarantees performance and quality.
Dendritic cells derived from human monocytes are generated through the culture in vitro of peripheral blood mononuclear cells and differentiation in the presence of GM-CSF and interleukin-4. They have a distinct dendritic shape, making them accessible to T cells. Centrally located within human immune responses, they are concentrated in the peripheral tissues and lymphoid tissues. These cells are notably highly plastic and can thus evolve different functional states under different microenvironmental conditions. In function, they're specialized antigen-presenting cells that activate naive T cells, induce T cell proliferation and differentiation, and decide what type of immune reaction to initiate (Th1, Th2, etc). They also participate in immunoregulatory mechanisms, producing cytokines that alter how other immune cells function and move.
In the research, human monocyte-derived dendritic cells are widely utilized in studies of human immunology. They play an important role in studying the mechanisms of interaction between pathogens and dendritic cells during the study of infectious diseases and are valuable in the development of anti-infective treatments. In cancer, studying them for tumor cell immune evasion and immune activation helps to create novel tumor immunotherapies. For example, designing and manufacturing tumor vaccines with dendritic cells.
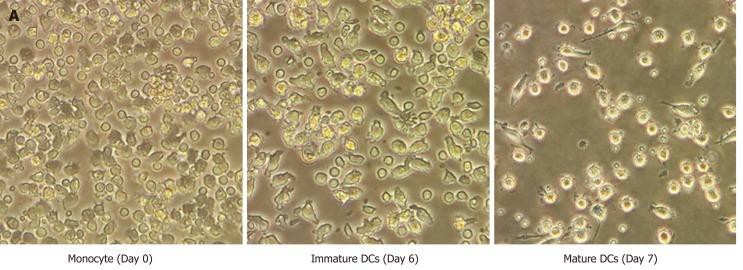 Fig. 1. Morphology of human monocyte-derived dendritic cells (Jiraviriyakul A, Songjang W, et al., 2019).
Fig. 1. Morphology of human monocyte-derived dendritic cells (Jiraviriyakul A, Songjang W, et al., 2019).
NOX2 Assembles in the Phagocytic Cup of Monocyte-Derived Dendritic Cells and Rapidly Produces ROS
Reactive oxygen species (ROS) are crucial in both pathogen elimination and antigen presentation. Neutrophils exhibit short, intense bursts of ROS mainly for pathogen killing. Whereas dendritic cells sustain lower ROS production rates, essential for antigen cross-presentation and adaptive immunity activation. However, the exact ROS production rates in dendritic cells, unlike neutrophils, have not been quantified. Therefore, Paardekooper's team aimed to quantify ROS production in human monocyte-derived dendritic cells by measuring their oxygen consumption rate during phagocytosis.
When neutrophils, macrophages, or dendritic cells engulf a pathogen, they quickly activate the NOX2 protein complex to generate superoxide anions within the phagosome. Hence, Paardekooper et al. examined the timing of NOX2 assembly post-phagocytosis. Immunofluorescence labeling showed overlap of NOX2 components, gp91phox and p67phox, at the phagosomal membrane just 5 minutes after zymosan addition, indicating rapid NOX2 assembly (Fig. 1A). Further microscopy using FITC-conjugated zymosan revealed NOX2 formation before phagocytic cup closure (Fig.1B-E), suggesting NOX2 activation occurs swiftly upon pathogen contact. Supporting this, about 75% of early endosomal marker EEA1-positive phagosomes exhibited gp91phox (Fig. 2). For insights on ROS production timing after zymosan uptake, we co-incubated dendritic cells with fluorescent zymosan and NBT, observing formazan crystal formation within 2 minutes of contact (Fig. 1F), continuing for at least 25 minutes (Fig. 1G). Together, these results show that NOX2 is assembled already at the nascent cup of emerging phagosomes and ROS production occurs rapidly (i.e., within minutes) after zymosan attachment.
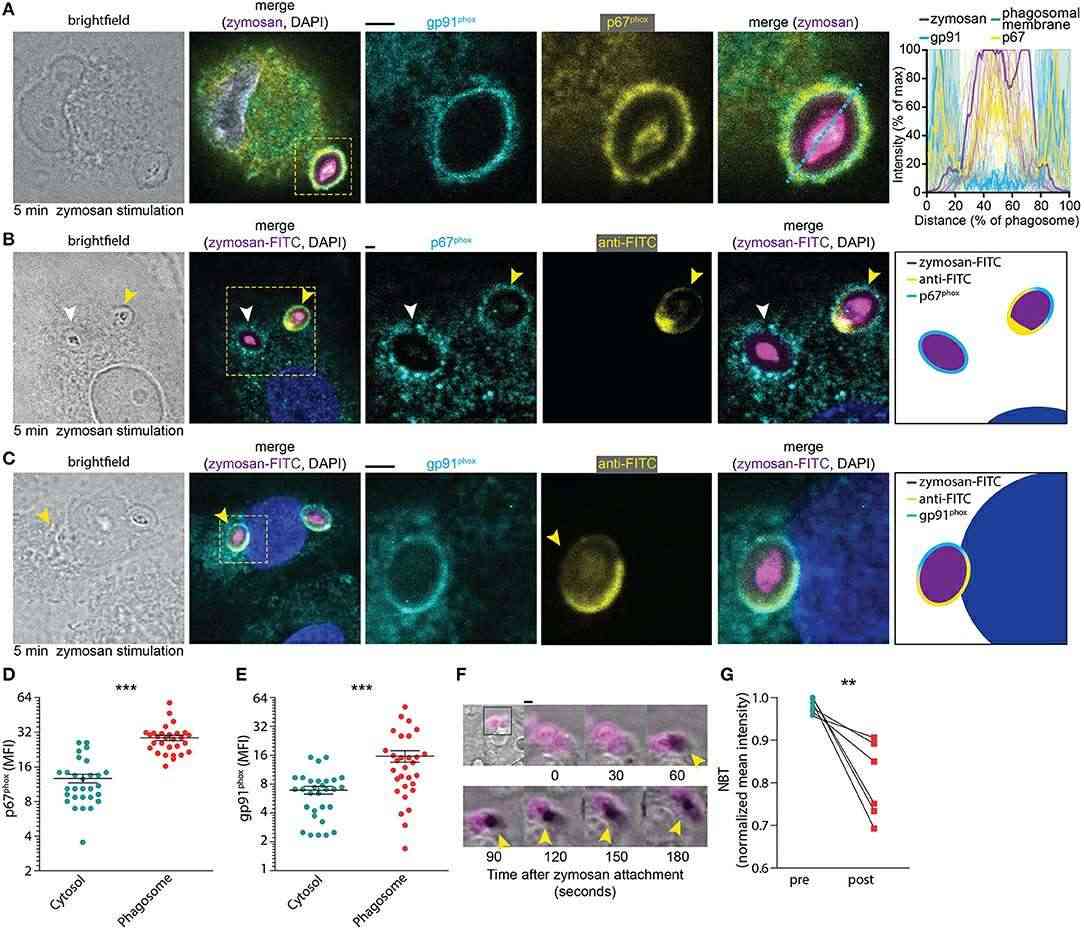 Fig. 1. NOX2 assembly at the phagocytic cup in monocyte-derived dendritic cells (Paardekooper LM, Dingjan I, et al., 2019).
Fig. 1. NOX2 assembly at the phagocytic cup in monocyte-derived dendritic cells (Paardekooper LM, Dingjan I, et al., 2019).
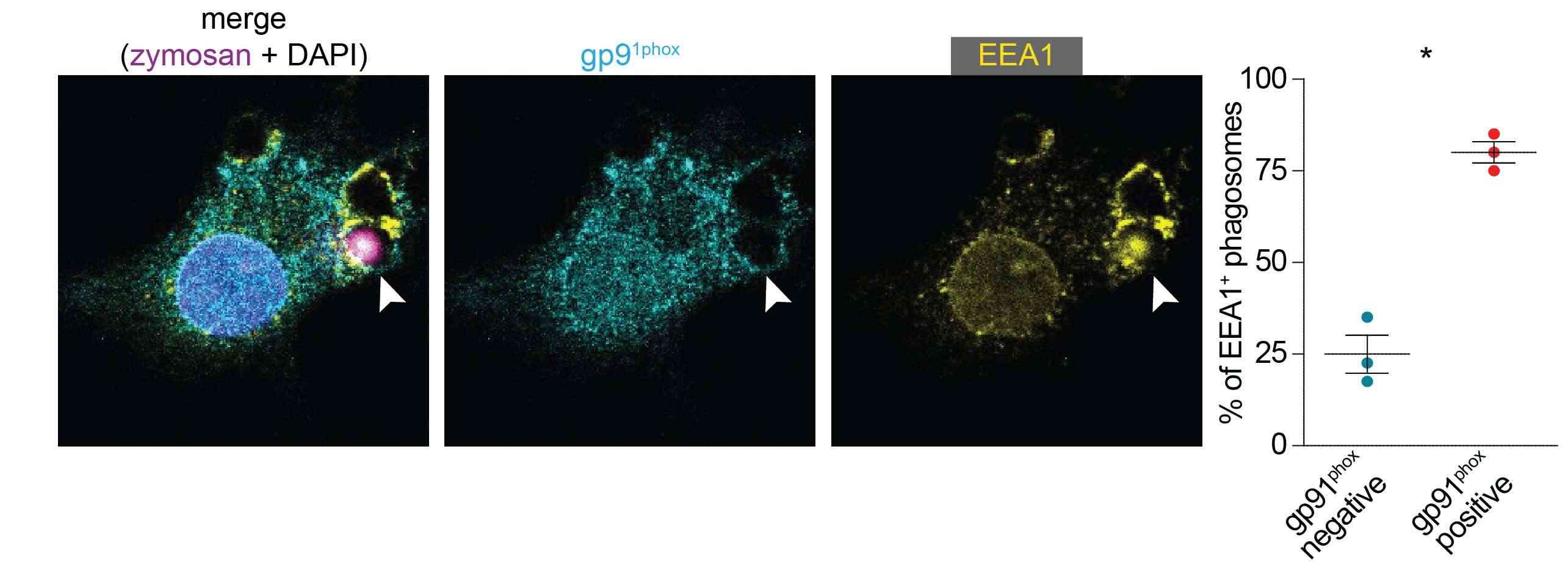 Fig. 2. gp91phox is present on EEA1-positive phagosomes (Paardekooper LM, Dingjan I, et al., 2019).
Fig. 2. gp91phox is present on EEA1-positive phagosomes (Paardekooper LM, Dingjan I, et al., 2019).
Toxicity towards Argon Plasma Treatment and ROS/RNS in Human Monocyte-Derived Dendritic Cells
Recent research suggests that cold physical plasma, which generates reactive oxygen and nitrogen species (ROS/RNS), can be used to treat cancer by its immunological effects. Antigen presentation and inflammation regulation are crucial in triggering antitumor immunity, with dendritic cells (DCs) being central to this process. However, the impact of plasma on DC inflammatory responses remains unclear.
Bekeschus's team aimed to explore the impact of plasma on human monocyte-derived DCs, assessing metabolic activity. They first tested the toxicity towards argon plasma treatment and ROS/RNS. The kINPen argon plasma jet was used for treating monocyte-derived dendritic cells (moDCs) 24 h (Fig. 3a). To assess sensitivity, moDCs and lymphocytes were treated together; dead cells were separately counted (Fig. 3c), revealing lymphocytes as more sensitive to argon plasma-induced cytotoxicity than moDCs (Fig. 3d). They then examined moDCs' metabolic activity and viability post-exposure to argon plasma or various ROS/RNS. Significant differences in metabolic activity were found when assessed kinetically after exposure to pilot ROS/RNS or plasma treatment (Fig. 4a), indicating detectable stress-induced activity changes. Endpoint experiments were performed at 24 hours after exposure to different durations of argon plasma (Fig. 4b) and concentrations of hydrogen peroxide (H2O2, Fig. 4c), hypochlorous acid (HOCl, Fig. 4d), peroxynitrite (ONOO, Fig. 4e), and lipopolysaccharide (LPS, Fig. 4f). With the exception of low-toxic LPS, all agents decreased their metabolic activity over time, consistent with flow cytometry viability measurements (Fig. 4g–k) and heatmap display (Fig. 4l).
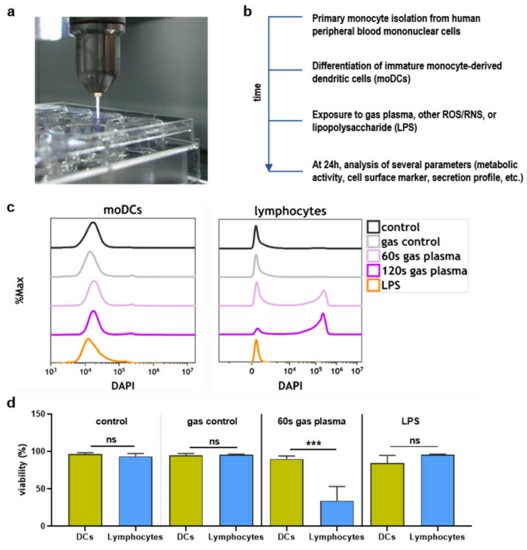 Fig. 3. Study protocol and toxicity comparison (Bekeschus S, Dorothee M, et al., 2021).
Fig. 3. Study protocol and toxicity comparison (Bekeschus S, Dorothee M, et al., 2021).
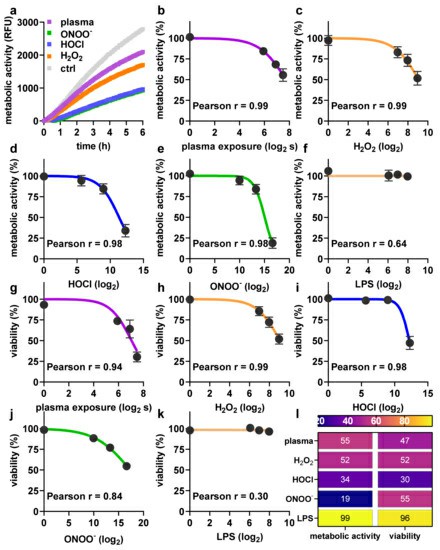 Fig. 4. Argon plasma treatment and reactive oxygen and nitrogen species (ROS/RNS) have dose-dependent toxicity profiles (Bekeschus S, Dorothee M, et al., 2021).
Fig. 4. Argon plasma treatment and reactive oxygen and nitrogen species (ROS/RNS) have dose-dependent toxicity profiles (Bekeschus S, Dorothee M, et al., 2021).
Ask a Question
Write your own review

