ONLINE INQUIRY

Human Retinal Astrocytes (HRA)
Cat.No.: CSC-7717W
Species: Human
Source: Retina; Eye
Cell Type: Astrocyte; Glial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The retinal astrocytes of the human eye are the retina's major glial cells and are essential to retinal tissue development during embryonic development. Retinal astrocytes in humans form a distinctive star shape, extending out into the inner plexiform layer of the retina. During development, these cells grow out of the head of the optic nerve, proliferate and expand into a reticular shape on the retinal surface. These progenitor cells, called astrocyte precursor cells (APCs), develop into premature perinatal astrocytes in early pregnancy.
In the human retina, astrocytes are predominantly found in the nerve fibre layer and the ganglion cell layer. They are spaced along the axis of nerve fibres, providing shelter and nourishment. They reside on ganglion cell surfaces and are closely related to ganglion cells, and are part of the fabric of the retina's intricate cellular environment. They also adorn the retinal blood vessels, and contribute to maintaining the shape and functional stability of the vasculature. In scientific studies, human retinal astrocytes are routinely used to investigate the onset, progression and pathophysiology of related retinal pathologies, including glaucoma and age-related macular degeneration. Usually, these diseases cause damage and loss of retinal neurons, and the role of astrocytes in disease progression is the focus of research.
The Effect of the LDN-0060609 PERK Inhibitor on HRA Cells with Activated ER Stress Conditions and mRNA Expression of ER Stress-Related Genes
Glaucoma refers to a group of neurodegenerative eye disorders with primary open-angle glaucoma being the most common. Human retinal astrocytes (HRA) play a critical role in its progression. Research shows that endoplasmic reticulum (ER) stress and PERK activation lead to HRA cell apoptosis, undermining neuroprotection. Rozpędek-Kamińska et al. assessed the effectiveness of the small-molecule PERK inhibitor, LDN-0060609, in countering ER stress in HRA cells, with the goal of developing a new anti-glaucoma treatment.
PERK phosphorylates eIF2α during ER stress. To assess LDN-0060609's effectiveness, p-eIF2α levels were measured via Western blot. HRA cells were preincubated with LDN-0060609 PERK inhibitor (0.75-50 μM) for one hour, then treated with 500 nM ER stress inducer Th for two hours. Controls included cells with 500 nM Th only and cells in complete culture medium. Th increased p-eIF2α in HRA cells significantly compared to controls. LDN-0060609 treatment reduced eIF2α phosphorylation in ER-stressed HRA cells, especially at 25 μM, inhibiting phosphorylation by 49% (Fig. 1). The mRNA levels of ATF4, DDIT3 (CHOP), BAX, and Bcl-2 were measured in HRA cells treated with LDN-0060609 (0.75 μM and 25 μM), both under normal and ER stress conditions. Cells treated with Th and 25 μM LDN-0060609 showed a significant reduction in the pro-apoptotic genes ATF4, DDIT3, and BAX compared to Th alone. Conversely, 25 μM LDN-0060609 in ER-stressed cells significantly increased anti-apoptotic Bcl-2 gene expression compared to untreated ER-stressed cells (Fig. 2).
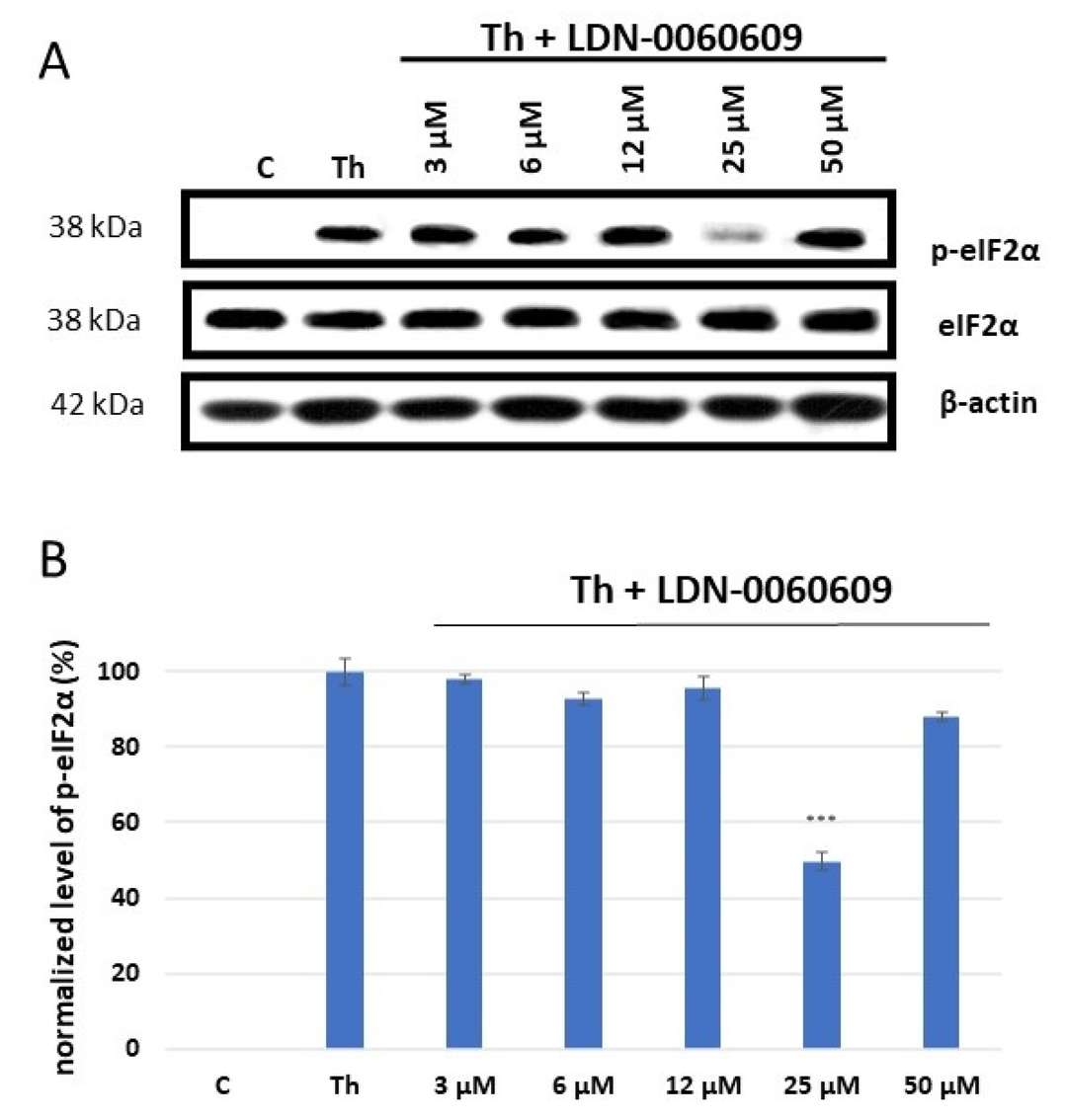 Fig. 1. Western blot analysis (A) and quantification measured by means of optical densitometry (B) of eIF2α phosphorylation in HRA cells (Rozpędek-Kamińska W, Galita G, et al., 2024).
Fig. 1. Western blot analysis (A) and quantification measured by means of optical densitometry (B) of eIF2α phosphorylation in HRA cells (Rozpędek-Kamińska W, Galita G, et al., 2024).
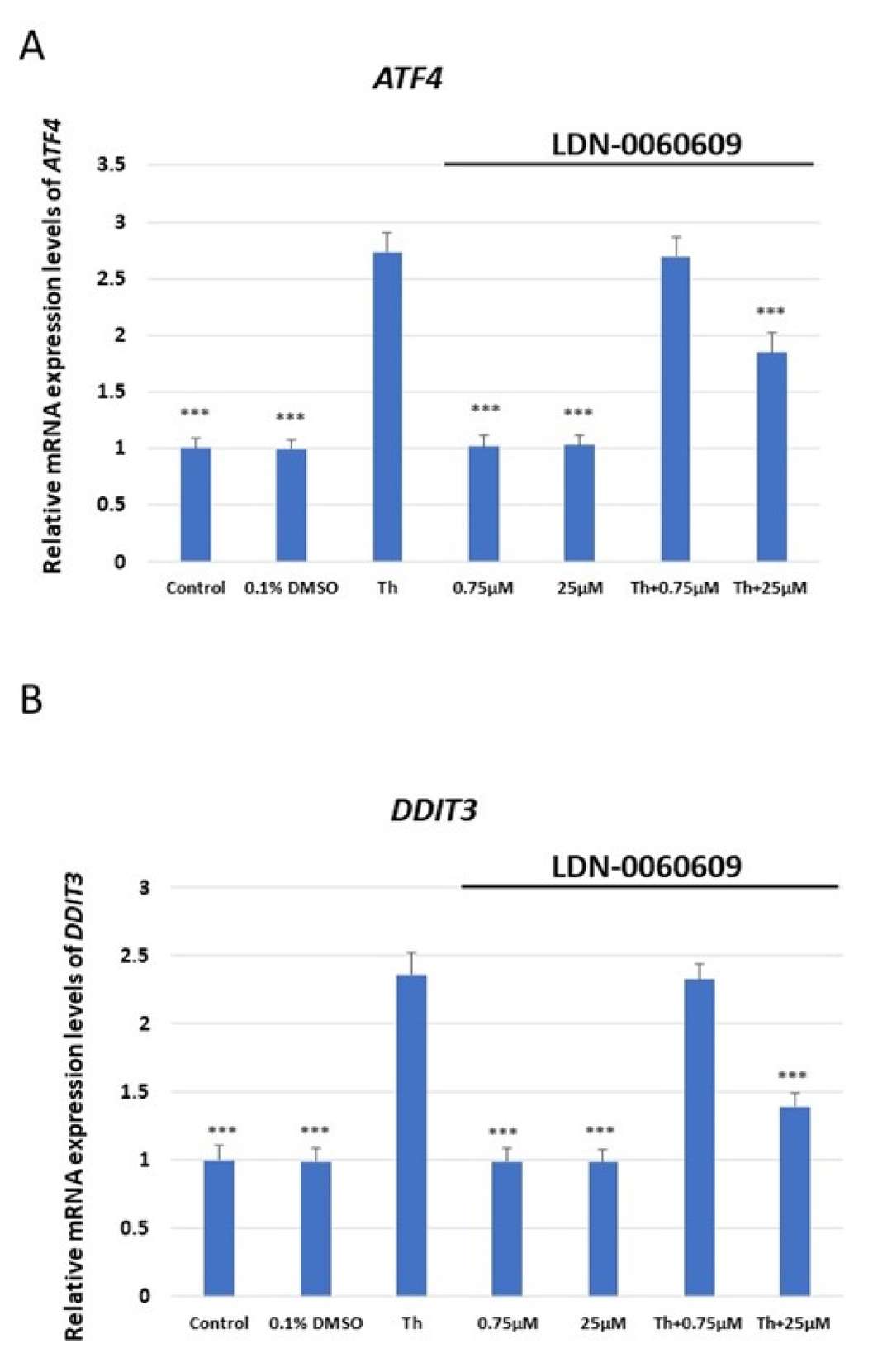 Fig. 2. Evaluation of the mRNA expression of the ER stress-related apoptotic genes: (A) ATF4, (B) DDIT3, (C) BAX, (D) Bcl-2 in HRA cells (Rozpędek-Kamińska W, Galita G, et al., 2024).
Fig. 2. Evaluation of the mRNA expression of the ER stress-related apoptotic genes: (A) ATF4, (B) DDIT3, (C) BAX, (D) Bcl-2 in HRA cells (Rozpędek-Kamińska W, Galita G, et al., 2024).
Inclusion of Perivascular Cell Types Improves Structural Properties of the iBRB-on-a-chip
Diabetic retinopathy (DR) is a microvascular disorder leading to vision loss due to inner blood-retinal barrier (iBRB) damage. Current models lack translatability to clinical DR, which hinders understanding of disease mechanisms and development of therapies. Maurissen et al. developed a diabetic iBRB-on-a-chip to replicate DR pathophysiology in vitro and explore potential drug targets, focusing on pericyte and endothelial cell interactions to inform novel therapeutic strategies.
They tailored the model for retina by co-culturingprimary human retinal microvascular pericytes (HRPs) and primary human retinal astrocytes (HRAs) in a 1:1:1 ratio within a fibrin gel, reflecting the pathological changes each cell type undergoes in retinal diseases. The chosen 1:1 EC to pericyte ratio reflects human retinal conditions, and a comparable astrocyte portion was included based on previous studies. Cultured with 50 ng/ml VEGF for 4 days, the system formed iBRB MVNs, transitioning to quiescence with reduced VEGF to 5 ng/ml from day 4 to 7 (Fig. 3a, Fig. 4a). Confocal images showed cell organization into networks mimicking iBRB with perfusable lumina (Fig. 3b, c, d, Fig. 4b, c). Mono-cultures of ECs had fewer interconnections, while co-culture with pericytes formed denser networks (Fig. 3e, f, Fig. 4d, e). Astrocytes reduced size and vascular area, indicating a regulatory role (Fig. 4f, g). Astrocyte and pericyte presence enhanced EC organization. Notably, using HRMVECs from different donors did not affect network formation, highlighting the robust nature of the tri-culture system. These results highlight that HRMVECs in our tri-culture system formed 3D MVNs independent of the donor.
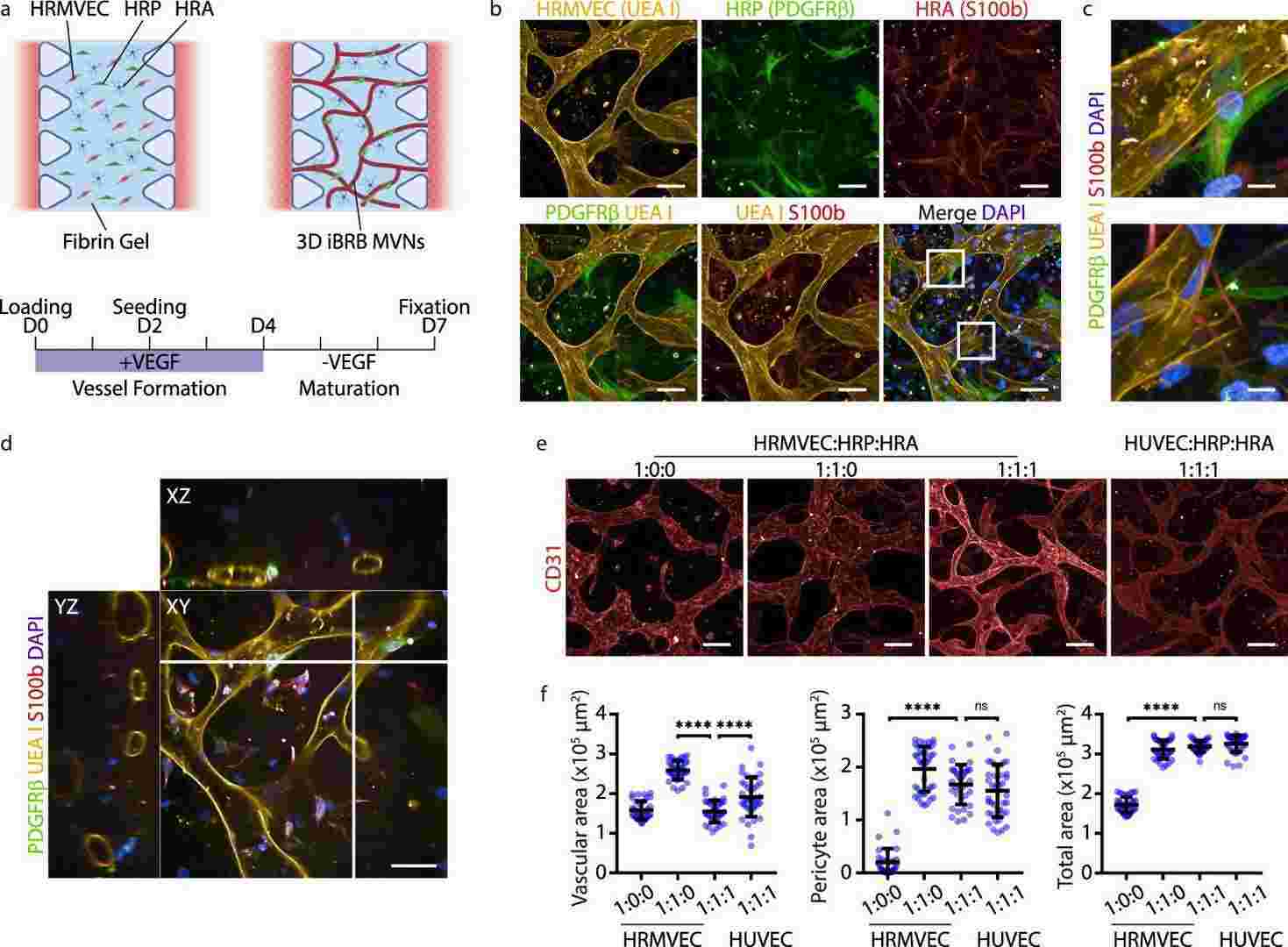 Fig. 3. Inner blood-retinal barrier model formed of self-assembled microvascular networks (Maurissen TL, Spielmann AJ, et al., 2024).
Fig. 3. Inner blood-retinal barrier model formed of self-assembled microvascular networks (Maurissen TL, Spielmann AJ, et al., 2024).
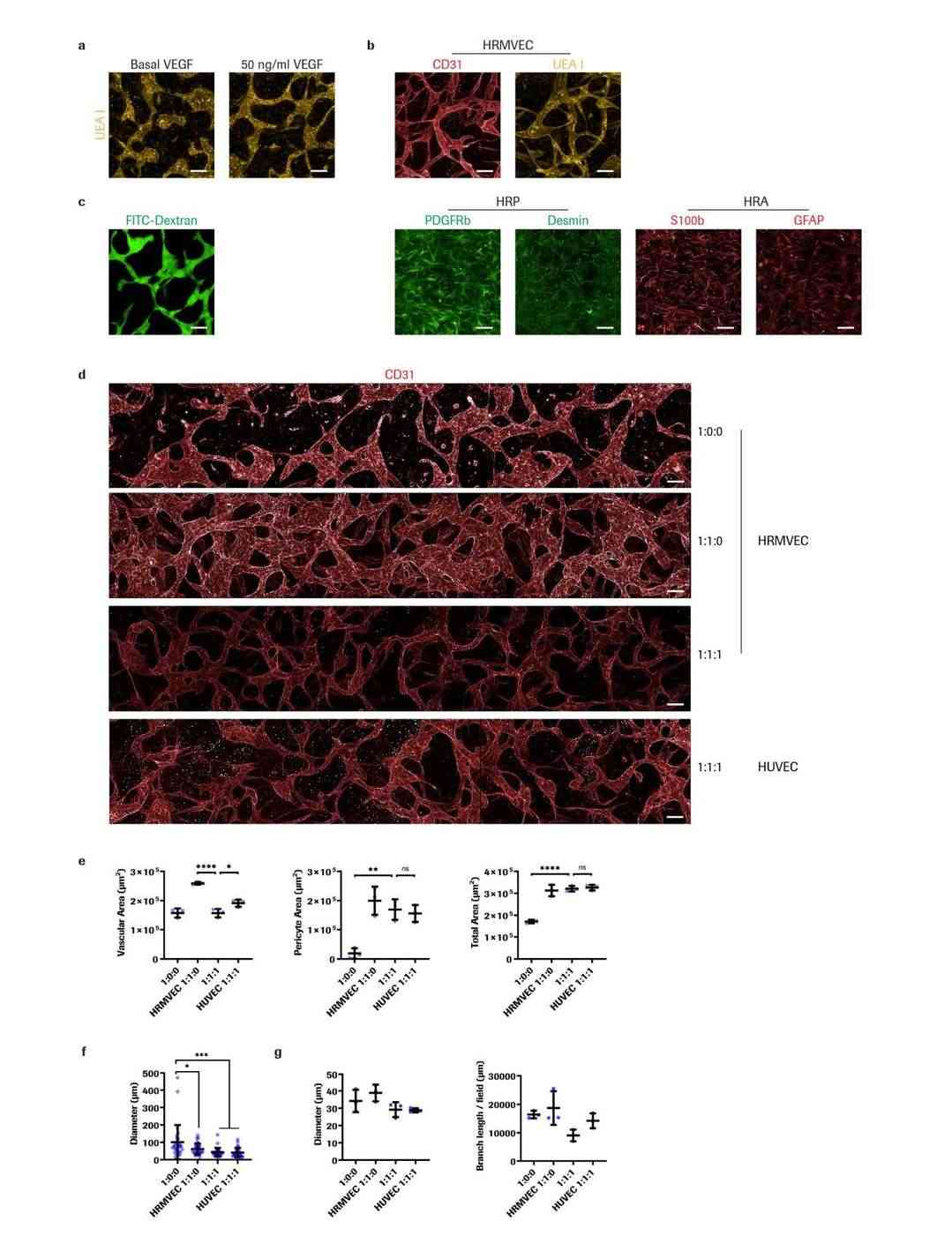 Fig. 4. Characterization of iBRB MVN morphology (Maurissen TL, Spielmann AJ, et al., 2024).
Fig. 4. Characterization of iBRB MVN morphology (Maurissen TL, Spielmann AJ, et al., 2024).
Ask a Question
Write your own review

