ONLINE INQUIRY

Human Liver Tumor-Associated Endothelial Cells
Cat.No.: CSC-C8585W
Species: Human
Source: Liver
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
The human liver tumor-associated endothelial cells (HLTECs) involved in liver cancer are a prime cause of the disease's development and progression. They build a separate population in the liver cancer tissue and form the tumor's blood vessels to provide nutrients and oxygen, which promotes the tumour's expansion and proliferation. HLTECs are vastly different from normal endothelial cells. Particularly, HLTECs are highly angiogenic, utilizing growth factors in the tumor microenvironment to promote neovascularization and enhance tumor perfusion. HLTECs are also resistant to chemotherapy and targeted therapy drugs, which has proven to be one of the most common reasons for failure in treating liver cancer. More importantly, HLTECs help to establish the tumor immune microenvironment by releasing immune-suppressive factors that suppress the immune response against the tumour.
As high-throughput and single-cell sequencing methods have improved, researchers have found that HLTECs are highly heterogeneous in gene expression, signalling and cell function. This finding has opened up new therapeutic targets and tactics for precision medicine. Given that HLTECs are not highly prevalent within tumor tissues and are challenging to isolate, these endothelial cell lines have become crucial resources for research and treatment. Moreover, the interactions between HLTECs and neighboring tumor cells are not fully understood. Research utilizing these cell lines can aid in deepening the understanding of liver cancer mechanisms, opening new avenues for treating the disease.
Osimertinib Effectively Inhibits HCC Angiogenesis in Primary Human Liver Tumor-Associated Endothelial Cells In Vitro.
Hepatocellular carcinoma (HCC) is one of the world's leading cancer deaths. An EGFR-dependent kinase inhibitor, osimertinib, approved for non-small cell lung cancer, has been shown to have anti-cancer activity. Huang's team examined the impact of osimertinib on HCC and angiogenesis, and explored how it might be synergistic with venetoclax, a selective Bcl-2 inhibitor. HCC angiogenesis was modelled using human liver tumour-associated endothelial cells (HLTEC). HLTEC and osimertinib were plated on a Matrigel matrix rich in exogenous factors and extracellular matrix. In the control group, HLTEC formed extensive and smooth tubular structures, whereas osimertinib-treated HLTEC failed to align and form a capillary network (Fig. 1A). Quantification showed that osimertinib inhibited HLTEC capillary network formation in a dose-dependent manner (Fig. 1B). Additionally, osimertinib induced apoptosis in HLTEC (Fig. 1C). A time course analysis showed that osimertinib failed to cause HLTEC apoptosis in 6 hours, but it started killing after 24 hours (Fig. 1D). This was thus ruled out in the absence of any mechanism by which osimertinib had its anti-angiogenic effect through apoptotic effects.
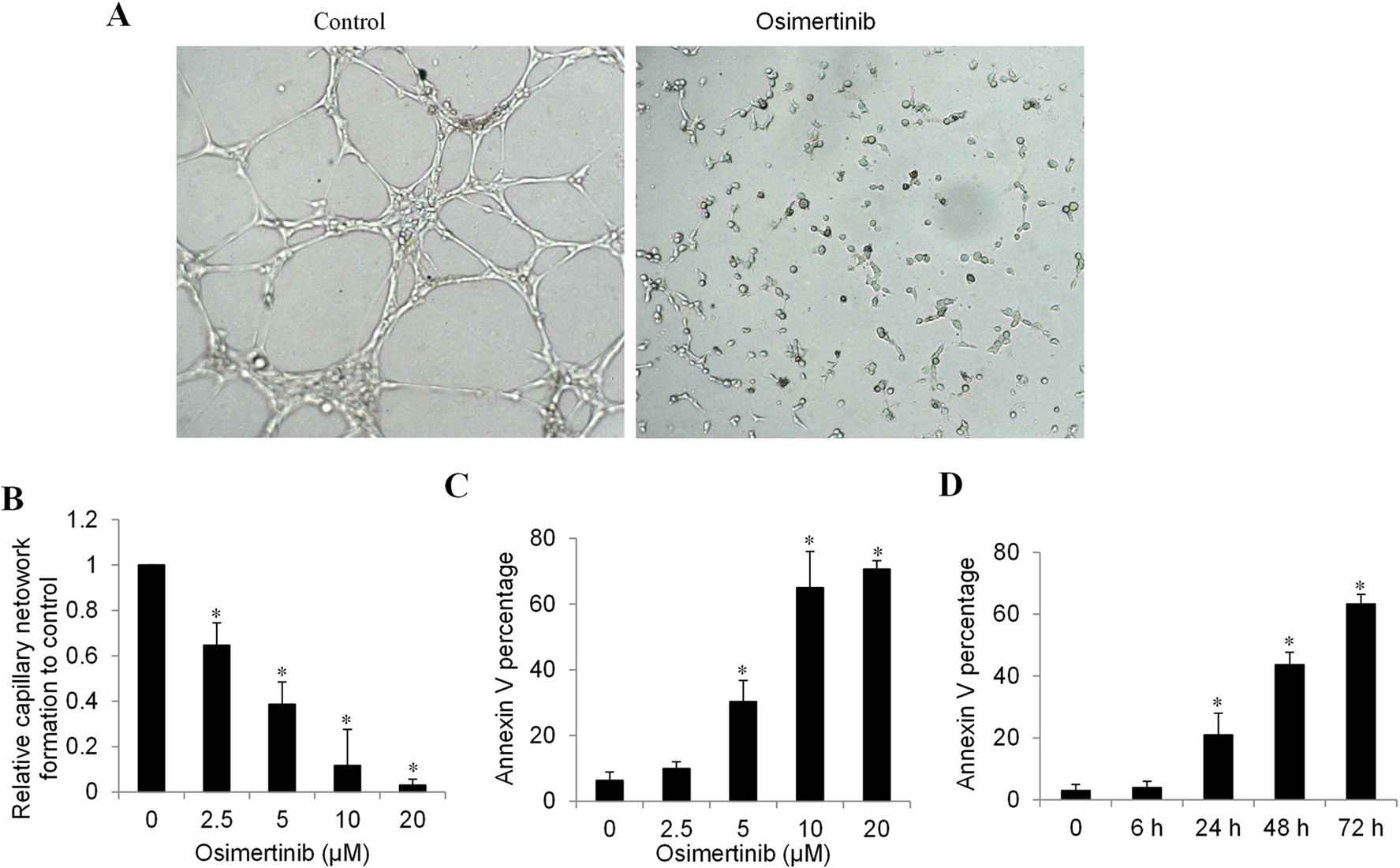 Fig. 1. Osimertinib inhibits HCC angiogenesis in vitro of primary human liver tumor associated endothelial cell (Huang, Q., He, S., et al., 2023).
Fig. 1. Osimertinib inhibits HCC angiogenesis in vitro of primary human liver tumor associated endothelial cell (Huang, Q., He, S., et al., 2023).
Genetic Difference of Sk-Hep1-Derived Tumor Tissue between Male and Female Mice
Estrogen is considered to be one of the factors influencing liver cancer, however, its role in liver cancer progression remains unclear. Oh, S. et al. investigated how estrogen influences the progression of liver cancer by transplanting SK-Hep1 (human liver tumor associated endothelial cells) into ovariectomized mice and examining the growth of tumor tissues after estrogen treatment (Fig. 2a). From the total RNA sequencing data, 15 genes, including KiSS-1 metastasis-suppressor, Golgi transport 1A, meiosis expressed gene 1 homolog, MMP7, oxidized low density lipoprotein, transmembrane protein 52B, GSG1, ABI family member 3, signal-regulatory protein gamma, hemopoietic cell kinase, RP11-219E24.1, BOC cell adhesion associated, lysosomal-associated membrane protein, C6orf15, and HECT, C2 and WW domain containing E3 ubiquitin protein ligase 1 were exclusively expressed in female-derived tumors (Fig. 2b). Among these genes, MMP7, GSG1, and C6orf15 significantly affected liver cancer patients' overall survival (Fig. 2c) and could serve as predictive biomarkers. Low MMP7 and high GSG1 and C6orf15 expression levels correlated with prolonged survival. Protein levels of these genes were higher in female tumors (Fig. 2d), indicating gene expression variability based on endogenous factors.
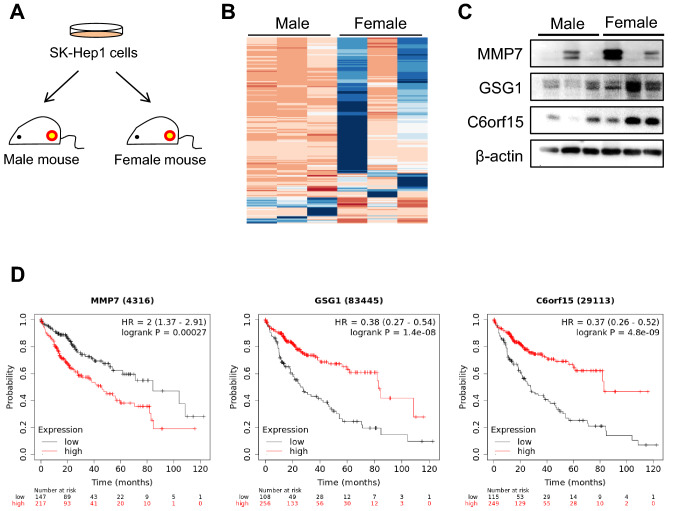 Fig. 2. Difference in gene expression between male and female mice bearing SK-Hep1-derived tumor (Oh S, Kwon HJ, et al., 2021).
Fig. 2. Difference in gene expression between male and female mice bearing SK-Hep1-derived tumor (Oh S, Kwon HJ, et al., 2021).
Ask a Question
Write your own review






