ONLINE INQUIRY

Human Liver Microsomes-200 Donors
Cat.No.: CSC-C4097X
Species: Human
Source: Liver
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Conduct studies of metabolic stability (% of original drug remaining over time)
Identify which cytochrome P450 (CYP) enzymes oxidize a drug candidate and which UDP-glucuronosyltransferase (UGT) enzymes are responsible for glucuronidation
Identify, quantify and characterize drug metabolites
Describe a drug's metabolic pathways
Identify inhibition of cytochrome P450 (CYP) enzymes
The human liver microsomes (HLM) are subcellular fractions created by disrupting liver tissue cells. They have a sausage-shaped or barrel-shaped, protected by a lipid bilayer that keeps them together and stable. Inside, they contain many of the enzyme proteins involved in cellular metabolism, which plays an important role both in the body and in vitro. Drug metabolism utilizes rich cytochrome P450 enzymes to oxidize, reduce and hydroxylate drug molecules to facilitates their elimination. In toxic metabolism, HLM participate in the removal of endogenous toxins by making them more water-soluble via metabolic changes, making them easier to be excreted. HLM are also involved in the metabolism of natural substances including lipids, cholesterol, and hormones. As such, HLM are used as in vitro models of drug metabolism and toxicity.
Creative Bioarray's human liver microsomes-200 donors are a mixture of liver microsomes from 200 different donors. Individual human liver microsomes can vary wildly in metabolic and enzyme activity. By collecting liver microsomes from multiple sources, the metabolic traits of individuals can be grouped together, which helps give a more accurate representation of the metabolic traits of the whole population. The resulting metabolic data is more stable and reproducible. Moreover, compared to conducting parallel studies using multiple single-donor samples, pooled HLM offer a simpler and more efficient method, saving considerable time and cost in experiments while simplifying experimental design and data analysis.
Determination of Contribution of CYP Enzymes in Mitragynine Metabolism Using rCYPs and HLM
Mitragynine is the major indole-based alkaloid of Mitragyna speciosa (kratom). Kratom has become increasingly utilized for mood elevation, pain treatment, and as a means of self-treating opioid addiction. There have been reports of deaths or toxic events associated with the use of kratom. However, information on the metabolic pathways and related enzymes of mitragynine is limited. Kamble et al. aimed to investigate the metabolic characteristics of mitragynine in human liver microsomes (HLM) (200 donors: 100 male and 100 female).
Kamble et al. first analyzed the metabolite profile of mitragynine in HLM using liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-QTOF-MS). Subsequently, they performed cytochrome P450 (CYP) reaction phenotyping using recombinant CYP enzymes (rCYPs) and HLM. When incubated with various CYP enzymes, mitragynine was significantly metabolized by rCYP3A4, with minimal metabolism by other CYPs (Fig. 1). Similarly, ketoconazole (a potent CYP3A4 and CYP3AS inhibitor) and CYP3cide (a selective CYP3A4 inhibitor) substantially inhibited mitragynine metabolism in HLM incubations (Fig. 2), indicating that CYP3A4 plays predominant role in the metabolism of mitragynine. In addition to studying mitragynine metabolism, they investigated how various CYP enzymes contribute to the formation of specific primary metabolites (Met2, Met4, Met5, Met6, Met7, Met8, and Met11) in both rCYPs and HLM. Ketoconazole and CYP3cide significantly inhibited the formation of Met2, Met4, Met5, Met6, and Met11 by 80%, indicating a major role for CYP3A4 in their formation (Fig. 1 and 2). Conversely, quinidine, a CYP2D6 inhibitor, significantly hindered the formation of Met7 and Met8, which aligns with rCYP2D6 data, suggesting CYP2D6's predominant role. The CYP2B6 inhibitor (PPP) also inhibited Met7 and Met8 formation due to cross-reactivity with CYP2D6.
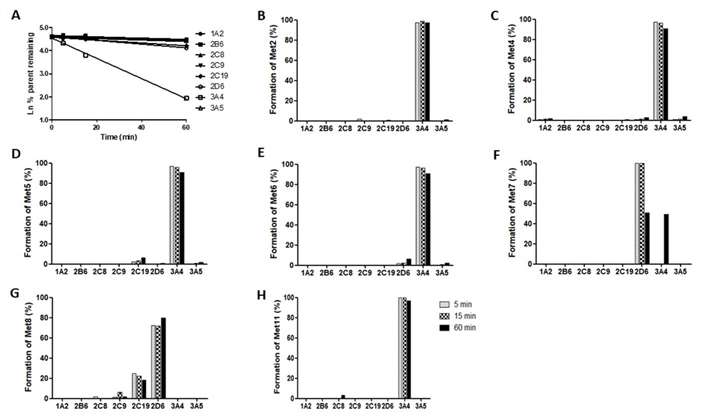 Fig. 1. The first order disappearance of mitragynine and metabolite formation (%) of mitragynine in the presence of various recombinant cytochrome P450 (rCYPs) (Kamble, S. H., Sharma, A., et al., 2019).
Fig. 1. The first order disappearance of mitragynine and metabolite formation (%) of mitragynine in the presence of various recombinant cytochrome P450 (rCYPs) (Kamble, S. H., Sharma, A., et al., 2019).
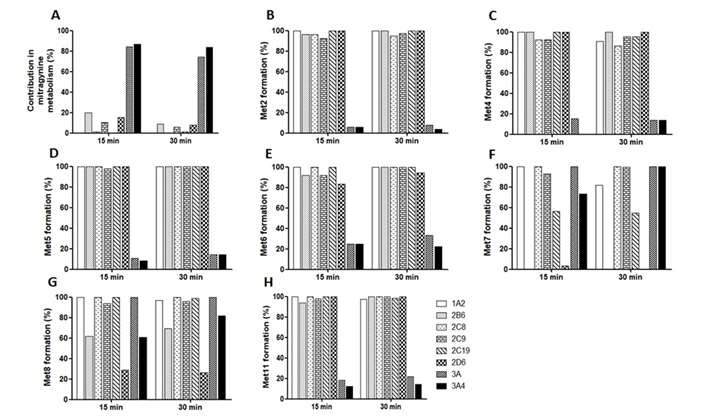 Fig. 2. Mitragynine metabolism and metabolite formation (%) of mitragynine in the presence of selective chemical inhibitors of CYPs in human liver microsomes (Kamble, S. H., Sharma, A., et al., 2019).
Fig. 2. Mitragynine metabolism and metabolite formation (%) of mitragynine in the presence of selective chemical inhibitors of CYPs in human liver microsomes (Kamble, S. H., Sharma, A., et al., 2019).
Identification of Metabolites of EPT, 4-OH-EPT, and 5-MeO-EPT after Incubation with pHLM
N-Ethyl-N-propyltryptamine (EPT), 4-Hydroxy-N-ethyl-N-propyltryptamine (4-OH-EPT), and 5-Methoxy-N-ethyl-N-propyltryptamine (5-MeO-EPT) are a class of psychoactive chemicals known as tryptamines. EPT has been reported to form metabolites via indole ring hydroxylation and other methods, but it is unknown how 4-OH-EPT and 5-MeO-EPT are converted. Most tryptamines are quick to break down, so it's important to pinpoint which metabolites will reveal intake. Therefore, Bergh et al. aimed to identify the major metabolites of EPT, 4-OH-EPT, and 5-MeO-EPT by incubating them with pooled human liver microsomes (pHLM).
These six or seven most prolific metabolites (EPT, 4-OH-EPT, and 5-MeO-EPT formed 60 min after incubation but could be detected at 240 min under pHLM) were identified as potentially useful metabolite markers for forensic analysis. Possible metabolite structures were explained by comparing the fragmentation of parent compounds with the metabolites. The seven most abundant metabolites were formed when EPT was incubated with pHLM, and the suggested structure is represented in Figure 3A. Figure 3B shows the metabolite profile from 5-MeO-EPT incubation with pHLM. Figure 3C shows the metabolite complex obtained by heating 4-OH-EPT with pHLM.
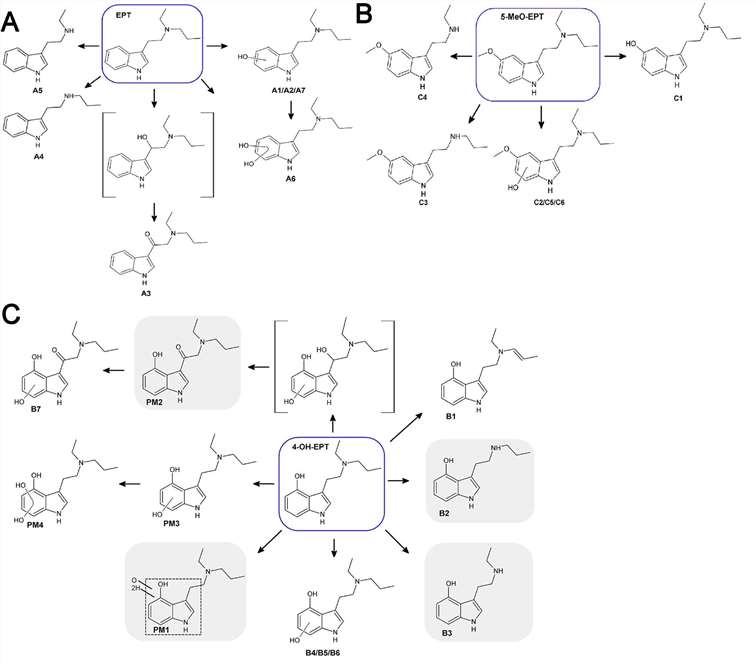 Fig. 3. Main metabolites of PET (A), 5-MeO-EPT (B) and 4-OH-EPT (C) identified after incubation with pHLM (Bergh M S, Bogen I L, et al., 2024).
Fig. 3. Main metabolites of PET (A), 5-MeO-EPT (B) and 4-OH-EPT (C) identified after incubation with pHLM (Bergh M S, Bogen I L, et al., 2024).
Concentration:20mg/ml
Yes, we also have pediatric liver microsomes from the single donor.
Ask a Question
Write your own review






