Human Liver Carcinoma Epithelial Cells
Cat.No.: CSC-C9218J
Species: Human
Source: Liver
Cell Type: Epithelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and is usually linked to HBV, HCV, liver cirrhosis, excessive alcohol consumption, chemical carcinogens such as aflatoxins and environmental causes. Human liver carcinoma epithelial cells or hepatocellular carcinoma (HCC) cells, which are originate from liver cancer tissues, have tumor cell line traits such as adhering growth. These cells usually have polygonal, round or oval epithelial shapes. They also carry out various specific liver tasks, such as the manufacture and release of plasma protein, cholesterol and triglyceride, the metabolism and transport of lipoproteins, the production of bile acids, the production of glycogen, and the action of insulin.
This cell line, as opposed to primary hepatocytes, has many advantages such as a limitless lifetime, uniform phenotype, high-availability, and easy handling. And it comes from pathological liver tissue of already diagnosed HCC patients, which is genetically very similar to those patients. It has developmental characteristics, functional protein expression, invasion and metastatic capabilities, and drug resistance almost identical to HCC patient cells. Also, its drug resistance profile resembles that of HCC patient cells, which makes it an invaluable marker for the efficacy and toxicity of therapeutic candidates. Therefore, it is widely employed as an alternative to primary hepatocytes for drug metabolism and hepatotoxicity research. It provides a solid framework for diagnosing the disease process from a genetic and cellular perspective to design and test new therapeutics. But it has limitations, including reduced expression of certain metabolic functions compared with hepatocytes, and should be evaluated in light of experimental requirements.
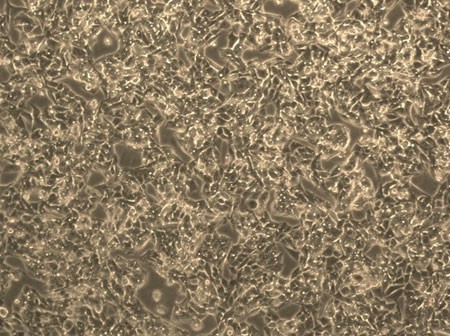 Fig. 1. Human liver carcinoma epithelial cells (HepG2) (Donato, M. T., Tolosa, L., et al., 2015).
Fig. 1. Human liver carcinoma epithelial cells (HepG2) (Donato, M. T., Tolosa, L., et al., 2015).
A HepG2 Cell-Based Biosensor That Uses Stainless Steel Electrodes for Hepatotoxin Detection
Environmental hepatotoxins can lead to liver failure. Biosensors may be the optimal candidates for detecting hepatotoxins. Live cells can serve as recognition elements in biosensors, but they may have difficulty adhering to the surface. This study investigated the suitability of HepG2 (hepatocellular carcinoma epithelial cells) as working electrodes in biosensors, assessing their adhesion capability on stainless steel surfaces. The sensor's performance was validated by detecting the viability of HepG2 cells exposed to different hepatotoxins.
Both the neutral red and coomassie brilliant blue cytotoxicity assays showed that cell viability decreased with higher concentrations of hepatotoxins (aflatoxin B1, AFB1 and isoniazid, INH). This indicates that both compounds exhibit hepatotoxic responses and can help assess the biosensor's limit of detection (LOD). Optical microscopy revealed a gradual decrease in HepG2 cell coverage with increasing AFB1 and INH concentrations, suggesting reduced tissue structure affinity. At higher concentrations, HepG2 cells underwent necrosis (Fig. 1). Fluorescence microscopy confirmed that in the HepG2 control group, many live, adherent cells were observed. As AFB1 concentration increased, live cell numbers decreased while dead cells increased, aligning with AFB1's hepatotoxic effects. For INH, dead cell numbers increased at 5 and 50 mM but were lower at 250 mM, possibly due to cell detachment or complete necrosis at high concentration. (Fig. 1b and c).
Electrochemical impedance spectroscopy (EIS) measurements were conducted to evaluate the efficacy of HepG2 cells adhered to a stainless steel surface as a biosensing device. For AFB1 assays, cells were almost completely shed. The Bode plot showed minimal overall impedance change, with significant phase differences below 1 Hz between the untreated control and treated biosensor, indicating no cell attachment (Fig. 2a and c). For INH testing, the difference between negative and positive controls was smaller, with slightly lower impedance due to potential increased conductivity from metabolites or necrosis products. Similar to AFB1, minimal impedance change was observed with slight differences among controls and samples (Fig. 2b and d). These results suggest that this biosensor can detect the presence of different hepatotoxins.
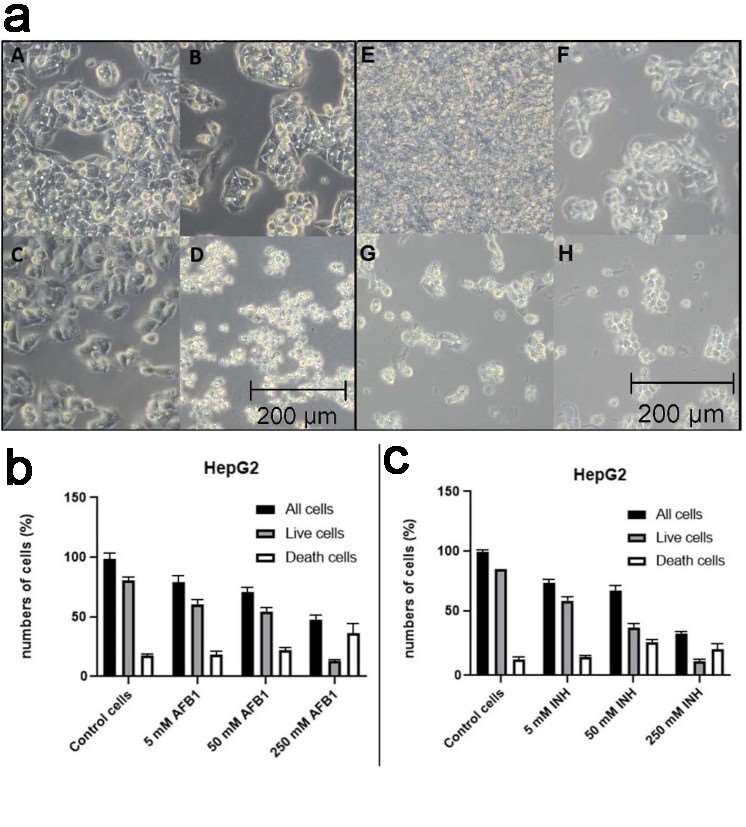 Fig. 1. Optical microscopy photographs under the influence of AFB1 on the HepG2 cell line (a) and impact of AFB1 and INH on HepG2 cell survival (b-c) (Rozman M., Štukovnik Z., et al., 2022).
Fig. 1. Optical microscopy photographs under the influence of AFB1 on the HepG2 cell line (a) and impact of AFB1 and INH on HepG2 cell survival (b-c) (Rozman M., Štukovnik Z., et al., 2022).
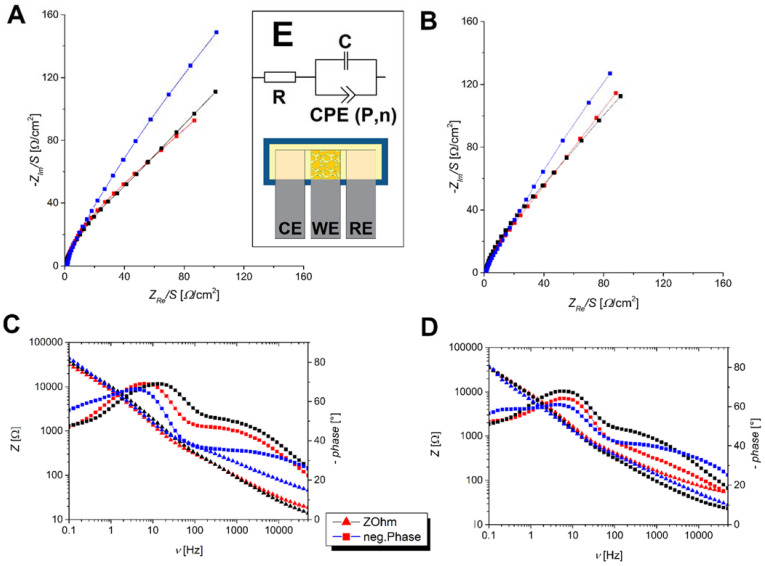 Fig. 2. Nyquist diagrams of investigated biosensors showing a response for aflatoxin B1 (A) and isoniazid (B), along with Bode diagrams for aflatoxin B1 (C) and isoniazid (D) (Rozman M., Štukovnik Z., et al., 2022).
Fig. 2. Nyquist diagrams of investigated biosensors showing a response for aflatoxin B1 (A) and isoniazid (B), along with Bode diagrams for aflatoxin B1 (C) and isoniazid (D) (Rozman M., Štukovnik Z., et al., 2022).
Lactonic Sophorolipid–Induced Apoptosis in Human HepG2 Cells through the Caspase-3 Pathway
Sophorolipids (SLs) are biosurfactants made by non-pathogenic yeast and secondary metabolites that microbes release in the right culture conditions. They are generally divided into lactonic sophorolipids (LSL) and acidic sophorolipids. LSL are the most biologically active among these and thus can be used as anti-cancer drugs due to its antibacterial, antiviral, and anti-inflammatory effects. Thus, Wang's team examined the effects of LSL on the in vitro growth of human liver carcinoma epithelial cells (HepG2 cells). The results indicated that LSL could suppress the growth of HCC cells via apoptosis.
To explore the molecular process that leads to LSL-induced apoptosis, they tested the activity of Caspase-3 and Caspase-9 in each group. They observed that Caspase-3 and Caspase-9 enzyme activity remained elevated in LSL and etoposide intervention groups (Fig. 3a and b). This indicated that LSL-induced apoptosis in HepG2 cells might be a function of the Caspase pathway. To determine how LSL caused apoptosis, the genes involved in apoptosis were identified in LSL-treated HepG2 cells and etoposide-treated cells. The levels of Apaf-1, Caspase-3 and Bax mRNA in both cells increased, and those of Bcl-2 mRNA decreased (Fig. 3c-f). To learn more about its functioning, they quantified the levels of apoptotic proteins. To get to know how it functioned, they measured the expression of apoptosis-related proteins. The experimental results identified that the expression of Caspase-3, Cleaved Caspase-3, Bcl-2, and Bax proteins in each group increased after the intervention of LSL and etoposide. The expression of Bcl-2 protein was reduced (Fig. 4). In conclusion, these results displayed that LSL changed related apoptosis genes and proteins in liver cancer cells, which further showed that LSL can induce apoptosis in liver cancer cells.
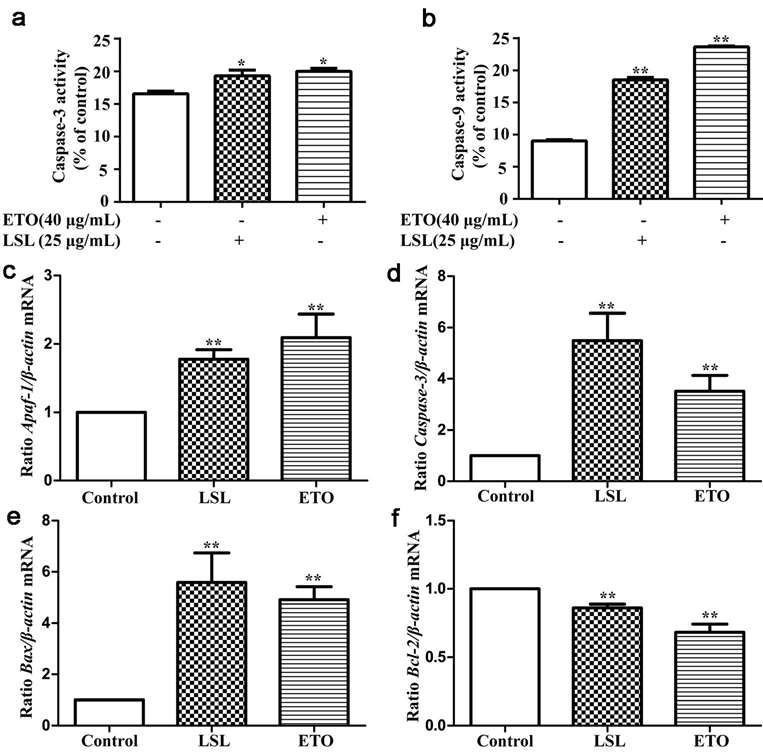 Fig. 3. Activity detection of Caspase-3 and Caspase-9 enzyme activity (a-b) and expression of related genes (c-f) after LSL and etoposide intervention in HepG2 cells (Wang, X., Xu, N., et al., 2021).
Fig. 3. Activity detection of Caspase-3 and Caspase-9 enzyme activity (a-b) and expression of related genes (c-f) after LSL and etoposide intervention in HepG2 cells (Wang, X., Xu, N., et al., 2021).
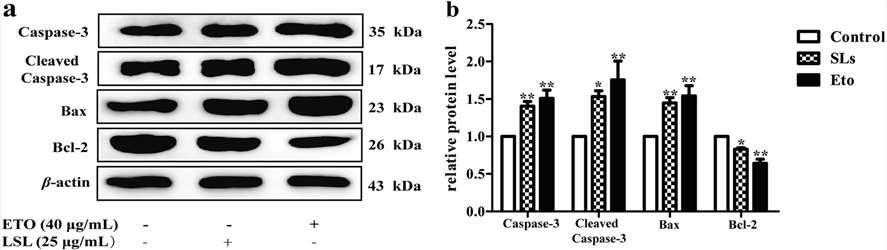 Fig. 4. Expression of related proteins after LSL and etoposide intervention in HepG2 cells (Wang, X., Xu, N., et al., 2021).
Fig. 4. Expression of related proteins after LSL and etoposide intervention in HepG2 cells (Wang, X., Xu, N., et al., 2021).
Ask a Question
Write your own review