ONLINE INQUIRY

Human Hepatic Stellate Cells
Cat.No.: CSC-C1496
Species: Human
Source: Liver
Cell Type: Hepatic Stellate Cell
- Specification
- Background
- Scientific Data
- Publications
- Q & A
- Customer Review
HHSteC are isolated from human liver. HHSteC are cryopreserved after purification and delivered frozen. HHSteC are characterized by immunofluorescent method with antibodies to desmin and α-actin. HHSteC are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. HHSteC are guaranteed to further expand for 15 population doublings in the conditions provided by Creative Bioarray.
Human hepatic stellate cells (HHSCs) were discovered by von Kupffer in 1876 as non-parenchymal liver cells. These cells are called "stellate cells" because they look star-like or spider-like with multiple, long cytoplasmic extensions. The HSCs are also called Ito cells, fat-producing cells, lipocytes or perisinusoidal cells. They are between the abluminal and basolateral sides of sinusoidal endothelial cells and hepatocytes, and represent 10–15 per cent of all liver cells. There are several biomarkers available for HHSC detection and isolation. Among these are ectodermal origin markers like NGF (nerve growth factor), nestin, synapsin and NCAM (neural cell adhesion molecule), and mesodermal origin markers like vimentin, desmin, α-smooth muscle actin (α-SMA), fibrillin, PDGFRβ (platelet-derived growth factor receptor β), PCDH7 (protocadherin-7), and hematopoietic markers.
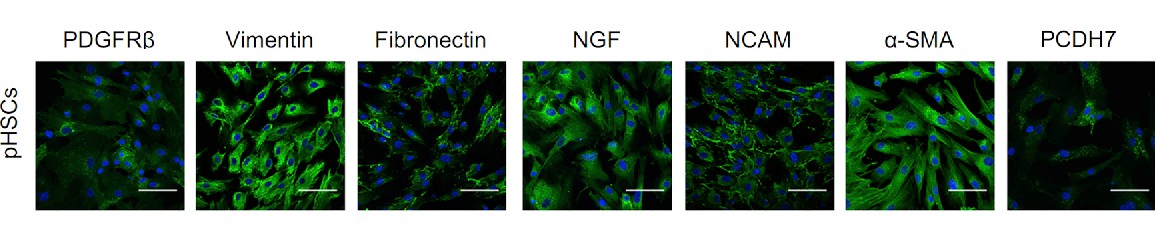 Fig. 1. Representative confocal images of immunofluorescences of PDGFRb, vimentin, fibronectin, NGF, NCAM, a-SMA, and PCDH7 in human primary HSCs. Scale bars, 100 mm (Coll, M., Perea, L., et al., 2018).
Fig. 1. Representative confocal images of immunofluorescences of PDGFRb, vimentin, fibronectin, NGF, NCAM, a-SMA, and PCDH7 in human primary HSCs. Scale bars, 100 mm (Coll, M., Perea, L., et al., 2018).
HHSCs in a normal liver are dormant, most of the time metabolizing and storing vitamin A, synthesizing and secreting elements of the extracellular matrix, and storing lipids (especially retinyl esters). But in response to pathological stimulus, HSCs multiply and convert into an activated type called myofibroblasts (MFBs). Highly active HHSCs have robust proliferation, production of α-SMA, less cytoplasmic retinol, and more cell membrane receptors. These inflamed cells secrete lots of extracellular matrix materials, which result in overproduction of ECM, and liver fibrosis. Additionally, HSCs can be stimulated by tumor cells to release growth factors and cytokines, which makes HHSCs closely linked with liver fibrosis or hepatocellular carcinoma (HCC). This means that HHSCs' biological function, regulation and disease correlation are necessary for elucidating liver disease and identifying novel therapies.
The Key Glycolytic Enzyme PFKFB3 is Up-Regulated During Hepatic Stellate Cell Activation and Its Blockade Attenuates Glycolysis
Activated hepatic stellate cells (HSCs) exhibit increased proliferation and matrix production. To meet these energy demands, they likely enhance aerobic glycolysis, driven by PFKFB3 (6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3), which produces fructose-2,6-bisphosphate (F2,6BP). The study began by characterizing the expression pattern of the critical glycolytic enzyme PFKFB3 during the activation of hepatic stellate cells (HSCs), which are primarily responsible for liver fibrosis.
Using freshly isolated mouse primary HSCs, they observed that PFKFB3 expression increased rapidly within 24 hours of culture-induced activation, confirmed by co-immunofluorescence with alpha-smooth muscle actin (α-SMA) (Fig. 1A and B). These results were corroborated in the human LX2 HSC line (Fig. 1A), a well-characterized model of human activated HSCs. LX2 cells were cultured for up to 7 days on plastic surfaces of cell culture dishes to mimic the molecular changes in HSCs during liver injury. Immunoblot analysis at different times demonstrated that PFKFB3 protein upregulation was an early and sustained event during HSCs activation, coinciding with increased expression of myofibroblastic markers, including α-SMA, platelet-derived growth factor (PDGF), PDGF receptor-ß, and collagen type I (Fig. 1C).
To investigate the biological effects of enhanced glycolysis, they examined the impact of the PFKFB3 small molecule inhibitor 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) on HSCs (Fig. 1D). LX2 cells were cultured for 3 days in a medium containing either a low dose of 3PO or vehicle (DMSO). Inhibition of PFKFB3 activity led to a significant 51% reduction in F2,6BP levels (Fig. 1D), a surrogate marker for glycolytic rate, without inducing cell death, consistent with previous studies. These findings suggest that HSCs activation involves a PFKFB3-regulated glycolytic reprogramming.
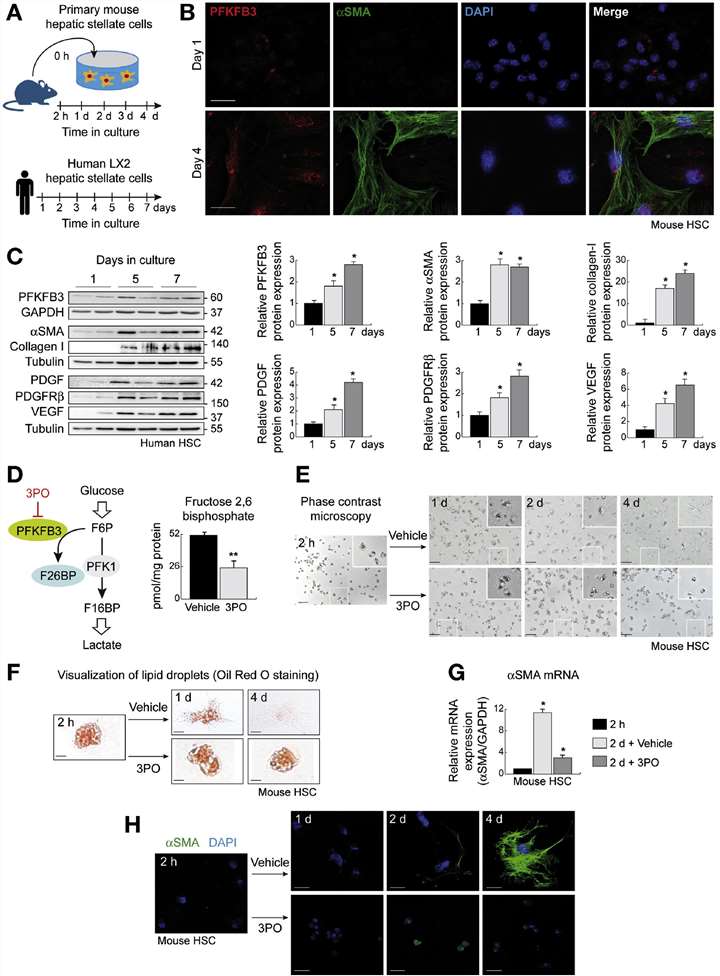 Fig. 1. PFKFB3 protein expression is up-regulated during HSCs transdifferentiation, and PFKFB3 blockade attenuates glycolysis in HSCs. (Mejias, M., Gallego, J., et al., 2020)
Fig. 1. PFKFB3 protein expression is up-regulated during HSCs transdifferentiation, and PFKFB3 blockade attenuates glycolysis in HSCs. (Mejias, M., Gallego, J., et al., 2020)
HSCs Can Express KIAA1363, and Pharmacological Inhibition of KIAA1363 Impairs RE Degradation upon Serum Starvation in LX-2 Cells and Primary Human HSCs
Large quantities of vitamin A are stored as retinyl esters (REs) in specialized liver cells, the hepatic stellate cells (HSCs). To date, the enzymes controlling RE degradation in HSCs are poorly understood. In this study, they identified KIAAl363 (also annotated as arylacetamide deacetylase 1 or neutral cholesterol ester hydrolase 1) as a novel RE hydrolase.
To investigate KIAA1363 expression in liver, they performed qPCR and Western blotting on total liver, primary murine hepatocytes, and HSCs. Purity of primary murine hepatocytes and HSCs was confirmed by high mRNA expression of the biomarkers (Fig. 2A). KIAA1363 showed highest mRNA expression in HSCs compared to primary hepatocytes and total liver. Western blot revealed KIAA1363 protein bands at ~50 kDa in HSCs, but not in total liver or primary hepatocytes (Fig. 2B). HSCs cultured on plastic surfaces can be activated and show high α-SMA expression. They examined KIAA1363 mRNA levels in quiescent HSCs and found an approximately 4-fold increase in KIAA1363 mRNA levels after 2 and 14 days of culture (Fig. 2C, left). Expression of α-SMA was initially low in resting HSCs and increased with time in culture (Fig. 2C, middle), contrasting with Lrat mRNA, which decreased (Fig. 2C, right).
Since they observed that KIAA1363 contributes to RE hydrolysis in murine HSCs, they next asked whether this was also conserved in human HSCs including LX-2 and primary human HSCs. Western blot analysis showed KIAA1363 expression in LX-2 cells but not in HepG2 cells (Fig. 3A). Using the KIAA1363-specific inhibitor JW480 and general serine hydrolase inhibitor Orlistat, they found that JW480 significantly reduced RE hydrolase activity by 80% in LX-2 cells (Fig. 3B). In primary human HSC lysates, JW480 led to a 92% reduction in RE hydrolase activity, while Orlistat had no effect (Fig. 3C). Pulse-chase experiments showed that JW480 completely inhibited RE degradation in LX-2 cells and primary human HSCs, whereas Orlistat had no significant impact (Fig. 3D). Similarly, in primary human HSCs, no detectable RP was found under basal conditions. Loading HSCs with ROH and oleic acid increased RP, which decreased by 83% after serum starvation. Orlistat had no effect on RP degradation, while JW480 resulted in 4-fold higher RP levels compared to DMSO control (Fig. 3E). These findings indicate that KIAA1363 plays a major role in RE hydrolase activity and metabolism in human HSCs.
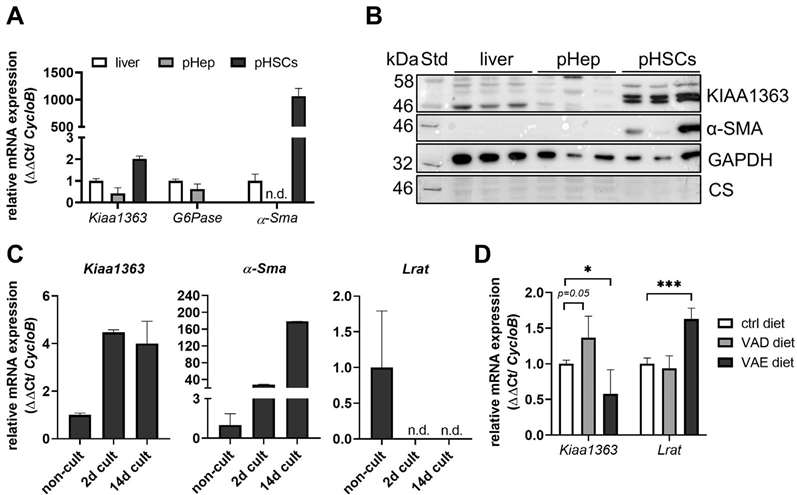 Fig. 2. KIAA1363 exhibits low expression in whole liver but is highly expressed in primary murine HSCs (Wagner, C., Hois, V., et al., 2022).
Fig. 2. KIAA1363 exhibits low expression in whole liver but is highly expressed in primary murine HSCs (Wagner, C., Hois, V., et al., 2022).
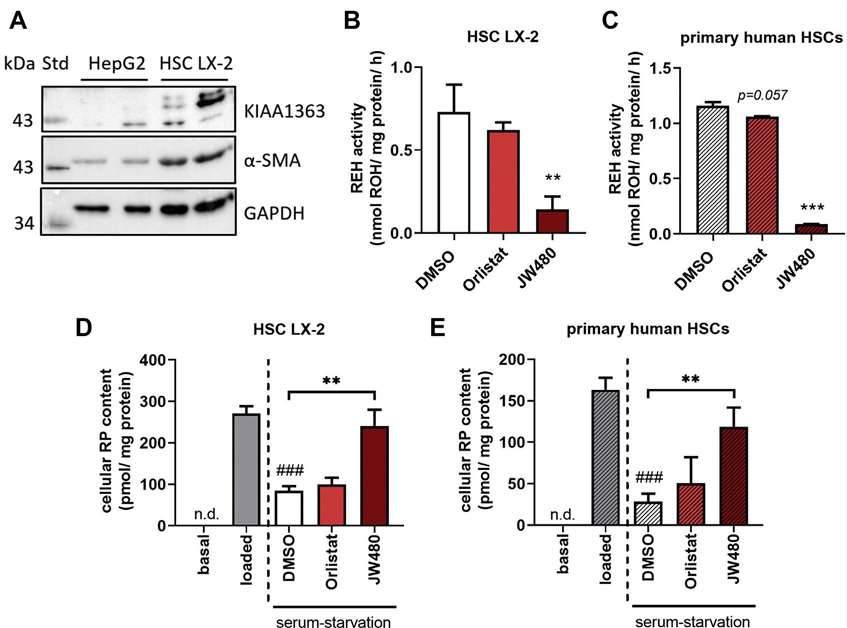 Fig. 3. Pharmacological inhibition of hKIAA1363 impairs RE degradation in LX-2 cells and primary human HSCs upon serum starvation (Wagner, C., Hois, V., et al., 2022).
Fig. 3. Pharmacological inhibition of hKIAA1363 impairs RE degradation in LX-2 cells and primary human HSCs upon serum starvation (Wagner, C., Hois, V., et al., 2022).
We can offer human hepatic stellate cells from healthy donors, but we do not routinely offer cells of any type from fibrotic livers. Enzymatic digestion of this tissue is almost always very poor, yielding poor quality cells.
Ask a Question
Write your own review






