ONLINE INQUIRY

Human Hepatic Sinusoidal Endothelial Cells
Cat.No.: CSC-C1494
Species: Human
Source: Liver
Cell Type: Endothelial Cell
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
HHSEC are isolated from human liver. HHSEC are cryopreserved immediately after purification and delivered frozen. Each vial contains >5 x 10^5 cells in 1 ml volume. HHSEC are characterized by immunofluorescent method with antibodies to vWF/Factor VIII and CD31 (P-CAM). HHSEC are negative for HIV-1, HBV, HCV, mycoplasma, bacteria, yeast and fungi. HHSEC are guaranteed to further expand for 15 population doublings in the conditions provided by Creative Bioarray.
Liver sinusoids regulate liver metabolism, including bilirubin metabolism, lipid production and secretion, plasma lipoprotein levels, and carbohydrate metabolism. Human hepatic sinusoidal endothelial cells (HHSECs), the endothelial cells that cover the liver sinusoids, are the largest number of non-parenchymal cells in the liver. These cells are very similar in their phenotype to dendritic cells and create dense, permeable fenestrae that allow the substances in the blood to efficient exchange between hepatocytes and the bloodstream. Additionally, HHSECs regulate a wide range of physiological processes, including vascular tone, liver metabolism and receptor-mediated elimination of endotoxins, bacteria and other substances. Furthermore, they maintain the hepatic immune system by secreting cytokines and activating immune cell signaling.
In healthy physiological circumstances, HHSECs adapt to different physiological and pathological stimuli by rearranging their fenestration and function via vascular endothelial growth factor (VEGF) in response to environmental demands. Yet all liver cells, including HHSECs, become dysregulated in the presence of acute and chronic liver damage. This dysregulation causes phenotypic and functional abnormalities, including capillarisation of liver sinusoids, the secretion of vascular smooth muscle signals, and vasoconstriction. Capillarisation means that HHSECs lose their specialized identity, turning them into regular, non-specialized endothelial cells. Therefore, modifying the activities and activity of HHSECs could enhance hepatic microcirculation, enable liver regeneration and repair, and offer novel treatments for liver disease.
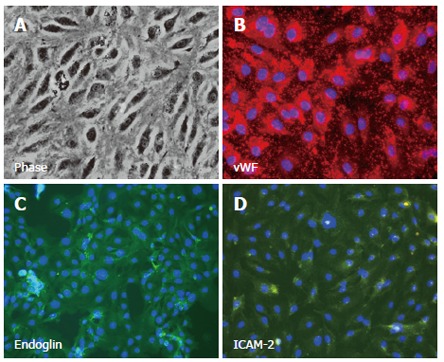 Fig. 1. Isolated, cultured human hepatic sinusoidal endothelial cells exhibit classical morphology under phase contrast microscopy (Lalor PF., Lai WK., et al., 2006)
Fig. 1. Isolated, cultured human hepatic sinusoidal endothelial cells exhibit classical morphology under phase contrast microscopy (Lalor PF., Lai WK., et al., 2006)
MIF Released by HHSECs Promotes the Migration Chemotaxis, EMT, Proliferation, and Apoptotic Resistance of CRC Cells
To determine the mediators released by Human hepatic sinusoidal endothelial cells (HHSECs) that induce colorectal cancer (CRC) cell migration, this study analyzed the supernatants from transwell chambers using cytokine arrays with 1000 antibodies. Notably, Macrophage migration inhibitory factor (MIF) was the most abundant in the conditioned media of HHSECs, especially in co-cultures with SW480 and HCT116 cells, compared to human umbilical vein endothelial cells (HUVECs) and the CRC cells alone (Fig. 1A and B). Application of either of two specific MIF inhibitors (ISO-1 and P425) resulted in the inhibition of HHSEC-induced migration of CRC cells. As a positive control, human recombinant MIF (rhMIF) was shown to enhance CRC cell migration. To evaluate MIF's chemotactic role from HHSECs, its expression was suppressed using lentiviral shRNA in HHSECs. The success of MIF knockdown and secretion inhibition was confirmed by qPCR, WB (Fig. 1C), and ELISA (Fig. 1D). Inhibition of migration induced by HHSECs was observed with the MIF inhibitor p425 or shMIF, and this effect was reversed by adding rhMIF (Fig. 1E).
When exposed to HHSEC-conditioned media, CRC cells developed a starfish shape with cytoplasmic extensions (Fig. 2A). The transwell assay showed these cells exhibited epithelial-mesenchymal transition (EMT) characteristics, including increased N-cadherin, vimentin, and decreased E-cadherin expression compared to non-migrated cells (Fig. 2B). Further, CRC cells cultured with HHSEC-conditioned media, which had higher soluble MIF, demonstrated increased mesenchymal markers and reduced E-cadherin, indicating MIF-induced EMT (Fig. 2C).
The CCK8 and EdU assays showed that high levels of MIF from HHSECs promoted the proliferation of SW480 and HCT116 CRC cells (Fig. 2D). Flow cytometry revealed that MIF increased the proportion of cells in the G2 phase without affecting G1 or S phases (Fig. 2E). Annexin V staining indicated that MIF reduced apoptosis in CRC cells, especially in those treated with 5-fluorouracil (5-FU) (Fig. 2F). Collectively, these data implied that MIF released by HHSECs activated EMT, proliferation, and apoptotic resistance during CRC cell migration.
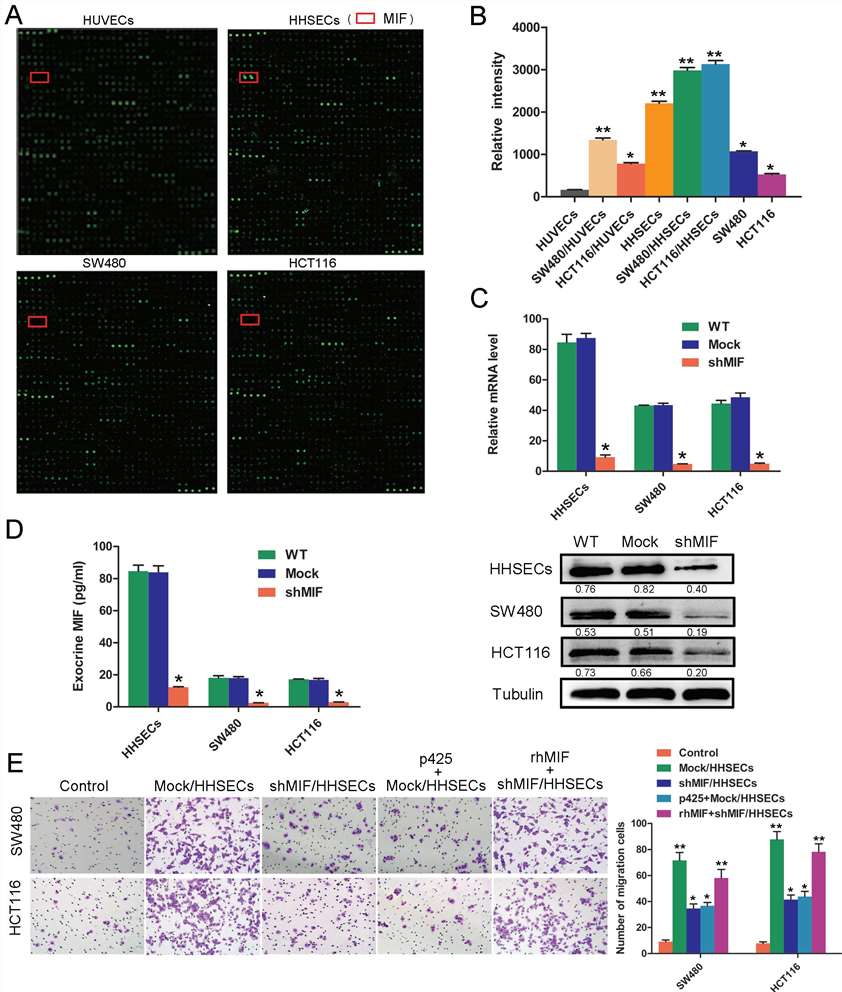 Fig. 1. MIF secreted by HHSECs is a critical factor for CRC cell migration (Hu, C. T., Guo, L. L., et al., 2015).
Fig. 1. MIF secreted by HHSECs is a critical factor for CRC cell migration (Hu, C. T., Guo, L. L., et al., 2015).
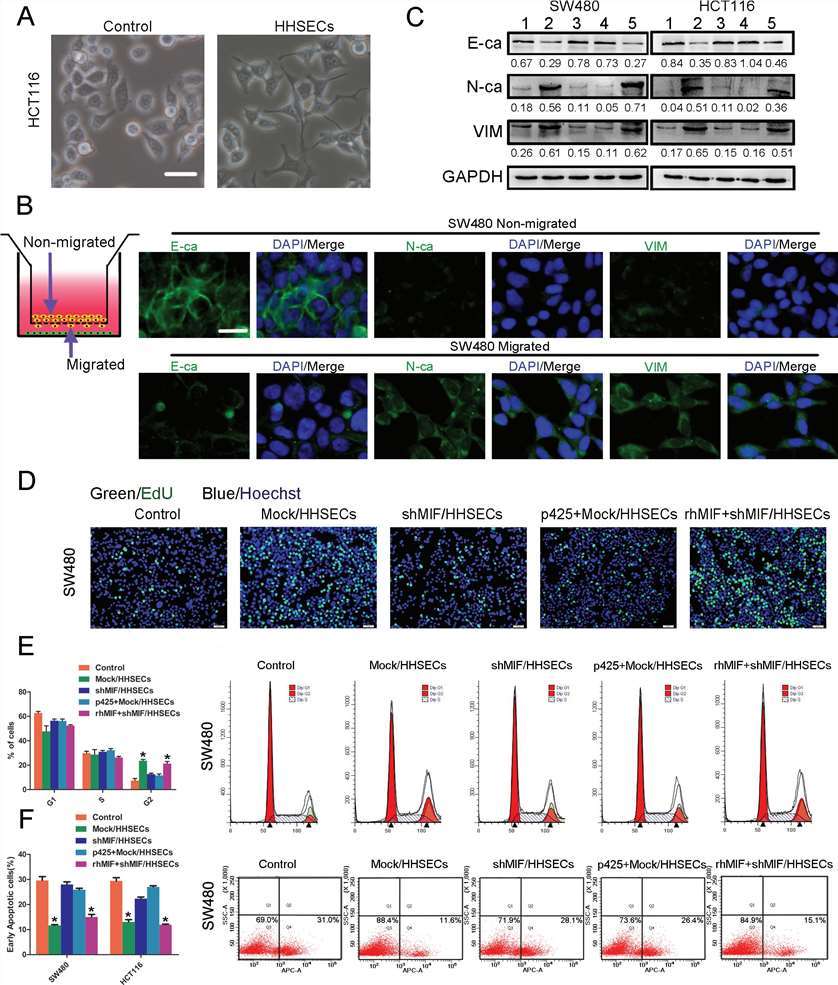 Fig. 2. MIF released by HHSECs promotes the EMT, proliferation, and apoptotic resistance of CRC cells (Hu, C. T., Guo, L. L., et al., 2015).
Fig. 2. MIF released by HHSECs promotes the EMT, proliferation, and apoptotic resistance of CRC cells (Hu, C. T., Guo, L. L., et al., 2015).
Long Non-coding RNA H19, a Negative Regulator of MicroRNA-148b-3p, Participates in Hypoxia Stress in HHECs Via NOX4 and eNOS/NO Signaling
H19 plays a controversial role in tumor occurring and progress. miR-148b-3p is one member of miR-148/152 family, which are differentially expressed in gastrointestinal tumors and participates in tumor growth, apoptosis, proliferation and angiogenesis drug susceptibility.
H19 targets miR-148b, blast analysis showed that miR-148b-3p was a potential regulator of NOX4 promoter but the function of miR-148b-3p has not been clarified in liver cirrhosis. Thus, this study investigates the participation of lnc H19, NOX4 and miR-148b-3p in cirrhotic patients and hypoxia stress in human hepatic sinusoidal endothelial cells (HHSEC), and clarifying the relationship among them. The results show that NOX4 mRNA and Inc H19 were upregulated, while miR-148b-3p was downregulated in cirrhotic patients and hypoxic HHSEC (Human hepatic sinusoidal endothelial cells). Hypoxia increased hydrogen peroxide and decreased eNOS/NO signaling in HHSEC.
To investigate miR-148b-3p's role in hypoxic stress in HHSEC, cells were transfected with miR-148b-3p inhibitor and mimics under hypoxic conditions for 24 hours. The results showed that hypoxia increased hydrogen peroxide levels and decreased nitric oxide levels. The miR-148b-3p inhibitor further increased hydrogen peroxide and decreased nitric oxide, while the mimics had the opposite effect. Additionally, hypoxia increased NOX4 and decreased p-eNOS protein levels, with these changes being enhanced by the inhibitor and reduced by the mimics (Fig. 3).
To investigate the regulatory roles of lncRNA H19 and miR-148b-3p, HHSEC cells were co-transfected with NOX4 overexpression and lncRNA H19 shRNA/miR-148b-3p inhibitor/miR-148b-3p mimics lentivirus vectors under hypoxia for 24 hours. The measurements showed that H19 shRNA reduced hydrogen peroxide production and NOX4 protein levels, effects reversed by co-transfection with miR-148b-3p inhibitor/NOX4 OE vectors. Similarly, miR-148b-3p mimics reduced hydrogen peroxide and NOX4 levels, also reversed by NOX4 OE transfection. H19 shRNA and miR-148b-3p mimics both increased nitric oxide content and p-eNOS levels, but these effects were weakened by NOX4 OE transfection (Fig. 4). In summary, lnc H19 is a negatively regulator of microRNA-148b-3p, and participate in hypoxia stress in HHSEC via positively regulating NOX4 and negatively regulating eNOS/NO signaling.
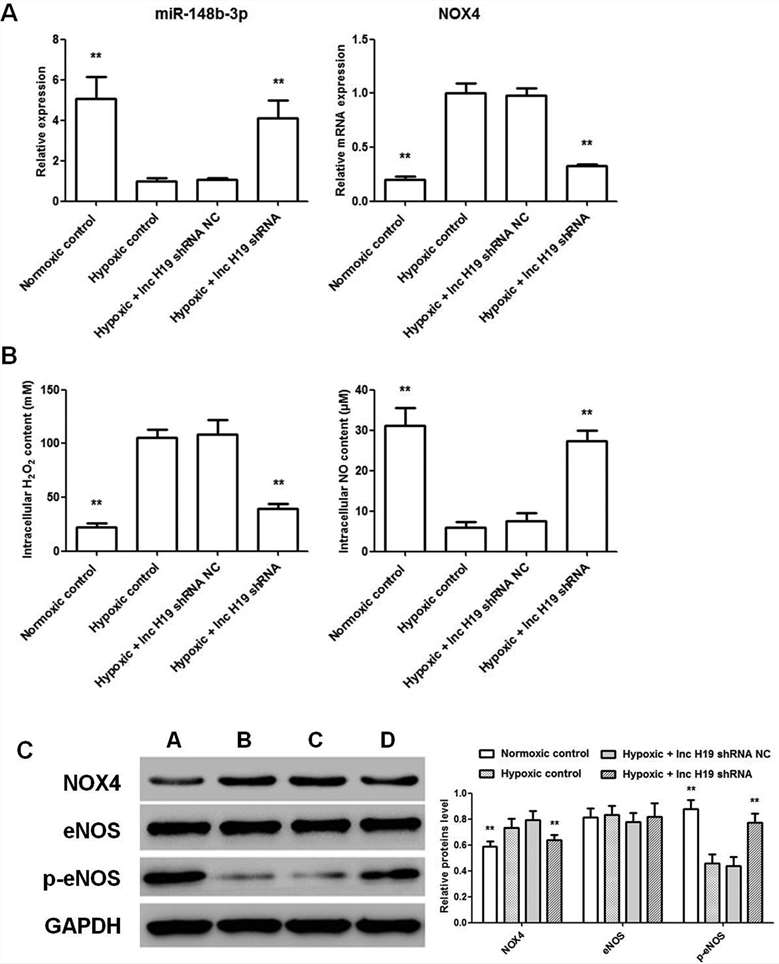 Fig. 3. Effect of miR-148b-3p inhibitor/mimics on HHSEC under hypoxic condition (Zhu, Y., Ni, T., et al., 2019).
Fig. 3. Effect of miR-148b-3p inhibitor/mimics on HHSEC under hypoxic condition (Zhu, Y., Ni, T., et al., 2019).
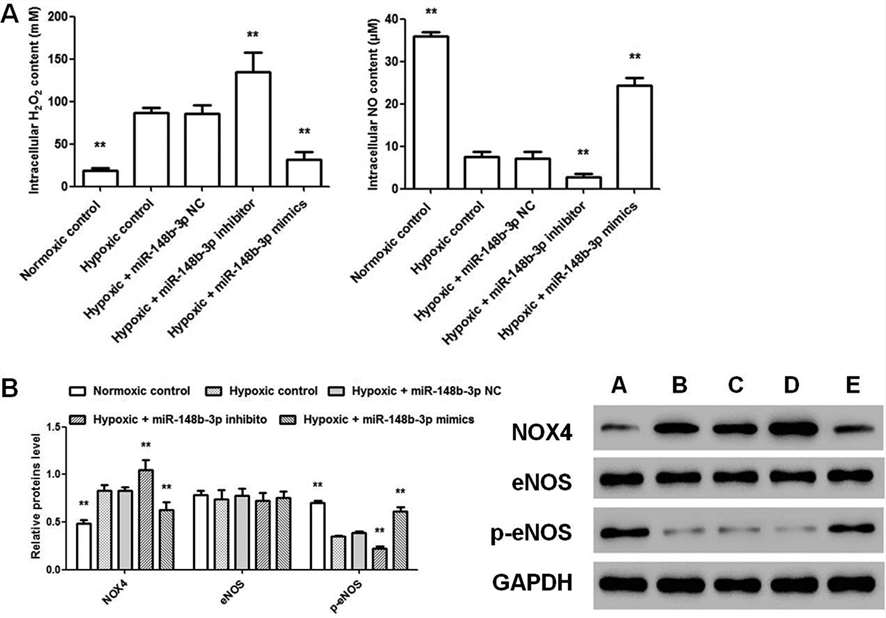 Fig. 4. Effect of combined treatment of NOX4 OE, lnc H19 shRNA and miR-148b-3p inhibitor/mimics on HHSEC under hypoxic condition (Zhu, Y., Ni, T., et al., 2019).
Fig. 4. Effect of combined treatment of NOX4 OE, lnc H19 shRNA and miR-148b-3p inhibitor/mimics on HHSEC under hypoxic condition (Zhu, Y., Ni, T., et al., 2019).
Regarding this product, please find below characterization info by ICC:
Cytoplasmic VWF / Factor VIII: >90% positive
Cytoplasmic uptake of Di-I-Ac-LDL: >90% positive
Cytoplasmic PECAM1: >90% positive
Ask a Question
Write your own review






